兩個基于依諾沙星的稀土配合物的合成、晶體結構、與DNA作用和抗菌活性
鄧記華 梅光泉
(江西省高等學校應用化學與化學生物學重點實驗室,宜春學院化生學院,宜春 336000)
兩個基于依諾沙星的稀土配合物的合成、晶體結構、與DNA作用和抗菌活性
鄧記華 梅光泉*
(江西省高等學校應用化學與化學生物學重點實驗室,宜春學院化生學院,宜春 336000)
通過溶劑熱-溶液揮發法合成得到了2個基于依諾沙星(HEx)的稀土配合物[Ce(Ex)4]·39H2O(1)和[Sm2(Ex)4(HEx)2(ox)]· 24H2O(2),并運用紅外光譜、元素分析和X-射線單晶衍射等研究手段表征了它們的結構。研究結果表明:配合物1為單核配合物;配合物2為雙核配合物。紫外滴定和熒光滴定研究結果表明:它們均能以插入的方式作用于小牛胸腺DNA,結合常數(Kb)分別為4.45×104和6.56×104mol-1·L;抗菌實驗結果表明它們對革蘭氏菌和真菌的活性均要優于依諾沙星配體本身。
依諾沙星;稀土配合物;晶體結構;DNA作用;抗菌活性
The study on the interaction of small metal coordinationmoleculeswith DNA isofgreat importance because it can provide important reference in the design of new and more efficient anti-cancer drugs.Over the past decades,a variety of transition metal complexes,especially Cu(Ⅱ),Ru(Ⅱ),and Pt(Ⅱ)complexes have been synthesized and studied for their interactions with DNA[1-3].Although recent researches show rare earth complexes have good biological activities on antibacterial,anti-inflammation,and sterilization[4],the report of their interactions to DNA are very limited[5].
Enoxacin(HEx)is an important type of quinolone antibacterial agent[6].It can penetrate into bacterial cells and then to inhibit activity of DNA gyrase[7].In this process,the HEx molecule bonding to ametal is considered as an important step[8].Therefore,it is meaningful to investigate the interaction between its metal complex and DNA.In this paper,we present two HEx-based rare earth complexes,[Ce(Ex)4]·39H2O (1)and[Sm2(Ex)4(HEx)2(ox)]·24H2O(2).Their synthesis,characterization,binding with CT-DNA,and antibacterial activity are investigated and discussed.
1 Experimental
1.1 Syntheses
1.1.1 Ce(Ex)4·39H2O(1)
Themixture of Ce(NO3)4·6H2O(1 mmol),HEx(2 mmol),and C2H5OH/H2O(16mL,V/V 1∶1)was placed in a 25 mL Teflon-lined reactor and heated at 110℃in an oven for 6 d.After cooled to room temperature, the resulting solution was filtered and the filtrate was allowed to stand at room temperature.Well-shaped light yellow crystals suitable for X-rays diffraction were obtained after one month with a yield of 53.6% based on HEx.Anal.Found(%):C,34.06;H,6.64;N, 10.51.Calcd.for C60H142F4N16O51Ce(%):C,33.99;H, 6.75;N,10.57.IR(KBr):ν(cm-1)3 396(s),2 773(w), 1629(s),1579(s),1474(s),1446(s),1 381(s),1 369(s), 1288(m),1256(m),1182(m),1128(w),968(w),825(m), 810(w),752(m),638(w),627(m),566(w),528(w),473(w). 1.1.2[Sm2(Ex)4(HEx)2(ox)]·24H2O(2)
The mixture of SmCl3·6H2O(1 mmol),HEx(2 mmol),Na2C2O4(0.5 mmol),NH3·H2O(1 mL),and C2H5OH/H2O(16mL,V/V 1∶1)was placed in a 25mL Teflon-lined reactor and heated at 120℃for 7 d. After cooled to room temperature,the resulting solution was filtered and the filtrate was left to stand at room temperature.Well-shaped light yellow crystals suitable for X-rays diffraction were obtained after two weeks with a yield of 61.7%based on HEx.Anal. Found(%):C,40.21;H,5.43;N,12.28.Calcd.for C92H146F6N24O46Sm2(%):C,40.34;H,5.37;N,12.27.IR (KBr):ν(cm-1)3 422(s),2 849(w),1 625(s),1 607(s), 1568(s),1474(s),1445(s),1367(m),1275(s),1 255(m), 1 143(w),1 114(m),1 092(w),943(w),822(s),790(w), 742(m),683(w),642(m),503(w).
1.2 X-ray structure determ ination
Single-crystal data for 1 and 2 were collected at 173 K on a Bruker Smart 1000 CCD diffractometer, with Mo Kαradiation(λ=0.071 073 nm),the empirical absorption correctionswere applied using the SADABS program[9].Both structures were solved using direct methods,which yielded the positions of all nonhydrogen atoms.These were refined first isotropically and then anisotropically.All the hydrogen atoms of the ligands were placed in calculated positions with fixed isotropic thermal parameters and included in the structure factor calculations in the final stage of fullmatrix least-squares refinement.The hydrogen atoms of lattice H2O molecules in 1/2 were not assigned.All calculations were performed using the SHELXTL system of computer programs[10].The crystallographic dataare summarized in Table 1.
CCDC:751128,1;751129,2.
1.3 Binding w ith CT-DNA
UV-Vis spectra of 1/2 in the absence and presence of CT-DNA were recorded on Varian Cary-100 UV-Vis spectrometer at room temperature.The absorption titrations of both complexes in buffer(5 mmol·L-1Tris-HCl,50 mmol·L-1NaCl,pH 7.2)were performed by fixing the complex concentration to which increments of the DNA stock solution were added,respectively.Both complex solutions employed were 20μmol·L-1in concentration,to which CT-DNA was added at a certain ratio cDNA/ccomplex.Complex-DNA solutions were allowed to incubate for 10 min before the absorption spectra were scanned at the range of 190~500 nm.
The interactions of the complexes 1/2 with CT-DNA were further investigated by the fluorescence method.As the ligand HEx itself can luminesce,the fluorescence measurements were directly carried out by adding small aliquots of a certain concentration DNA stock to a 20μmol·L-1complex in buffer[11].

Table 1 Crystal data and structure refinements for 1 and 2
1.4 Antibacterial tests
Antimicrobial activities of 1,2 and HEx as a reference substance,in 10%DMSO respectively,were evaluated using the agar diffusion test.The minimal inhibitory concentrations(MICs)of these compounds against six bacterial strainswere determined.
2 Results and discussion
2.1 Crystal structures
X-ray diffraction study indicates that 1 is a mononuclear complex.As shown in Fig.1a,the central metal Ce髧ion is eight-coordinated by eight oxygen atoms from four Ex-anions,forming a dodecahedron geometry.Each Ex-anion,employingbidentatechelating coordination mode,bonds to Ce髧through the carbonyl oxygen(O3)and carboxylate oxygen(O1). The bond distance of Ce1-Ocarbonyl(0.236 3(4)nm)is slightly longer than the value of the Ce1-Ocarboxylate(0.229 6(4)nm),which isconsistentwith the comparison result in other quinolone complexes[12].
2 is an ox2--bridged binuclear Sm(Ⅲ)complex. Each Sm(Ⅲ)ion,besides coordinatingwith two O atoms from one ox2-anion,also coordinates with six oxygen atoms from two Ex-anions and one HEx molecule, forming a square antiprism geometry.The ligands in 2, similar to those in 1,employ bidentate chelating coordination mode.The Sm-O bond distances,ranging from 0.236 2(3)to0.244 5(3)nm,areunexceptional.
2.2 DNA binding
To investigate the binding of 1/2 to CT-DNA,the absorption spectra of 1/2 in the absence and presence of CT-DNA were screened at 25℃.As shown in Fig. 2,with the addition of amounts of DNA,the MLCT band of 1 shifts from 276 to 282 nm and displays about 24%hypochromism(Fig.2a),and the MLCT band of 2 shift from 277 to 286 nm and yields 44% hypochromism(Fig.2b).These characteristics of red shift and hypochromism indicate that 1/2 binds with CT-DNAmostly using an intercalativemode[13].
In order to quantitatively calculate the binding of 1 and 2 to CT-DNA,the intrinsic binding constants Kbof 1 and 2 were determined from the decay of the MLCT band absorbance(inset in Fig.2)and calculated using the following equation[14].


Fig.1 ORTEP drawing of 1(a)and 2(b)with atomic numbering scheme

Fig.2 Absorption spectra of 1(a)and 2(b)in the presence of increasing amounts of CT-DNA with subtraction of the DNA absorbance
(

Fig.3 Emission spectra of 1(a)and 2(b)in the presence of increasing amounts of CT-DNA
The determined intrinsic binding constants for 1 and 2 are 4.45×104and 6.56×104mol-1·L respectively. These values are comparable to those of complexes reported in literatures[15].
The interactions of 1 and 2 with CT-DNA were further studied via emission spectra method.The results depicted in Fig.3 conspicuously illustrate the emission intensity of 1/2 increased steadily as increasing the amount of DNA,which implies 1/2 interacts with DNA mostly through an intercalative mode[16-17].This is quite consistent with the result concluded from the electronic absorption titration method.
2.3 Antibacterial tests
Table 2 shows the minimal inhibitory concentrations(MICs)of HEx,1 and 2 against six bacteria respectively.The testing results indicate that these compounds tested aremore active against Gram bacteria,but less active against fungi microbial (Candida albicans).Compared with the free HEx drug, the MIC value of 1 and 2 to any bacterial is smaller than the one of HEx,which reveals the 1 and 2 exhibit better activities than that of the free ligand. This may be ascribed to the synergic effect between themetal ions and HEx.
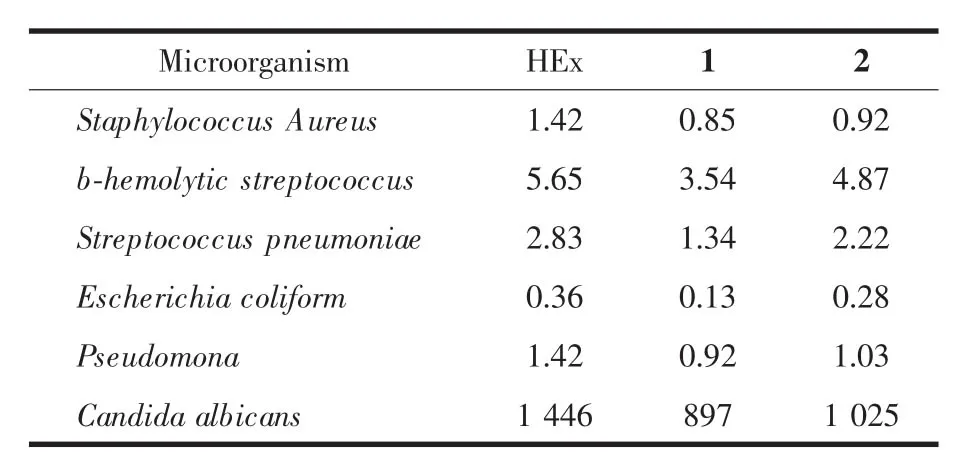
Table 2 M inimal inhibitory concentrations(M ICs,μg·m L-1)of HEx,1,and 2 for thefollow ing bacteria assayed
3 Conclusions
Two rare earth complexes based on HEx have been synthesized and characterized.X-ray single crystal diffraction analysis indicates that 1 is a mononuclear complex and 2 is a binuclear complex. DNA binding investigations indicate that 1 and 2 can moderately bind with double-stranded DNA via intercalative mode.Antibacterial tests reveal that 1 and 2 have better activity against bacterial than the free ligand HEx.
[1]Hostetter A A,Chapman E G,DeRose V J.J.Am.Chem. Soc.,2009,131:9250-9257
[2]Gao F,Chen X,Wang JQ,et al.Inorg.Chem.,2009,48:5599-5601
[3]HONG Xian-Lan(洪顯蘭),REN Jian-Min(任健敏).Chinese J.Inorg.Chem.(WujiHuaxue Xuebao),2011,27(4):785-790
[4]Ni JZ.Bioinorganic Chemistry ofRare Earth.Beijing:Science Press,2002.
[5]Wang Q,Yang ZY,QiG F,etal.Eur.J.Med.Chem.,2009, 44:2425-2433
[6]Well M,Naber-Kurt G,Kinzig-Schippers M,et al.Int.J. Antimicrob.Agents,1998,10:31-38
[7]Klopman G,Wang S,Jacobs M R,et al.Antimicrob.AgentsChemother.,1993,37:1799-1806
[8]Robles J,Martin Polo J,魣lvarez-Valtierra L,et al.Metal Based Drugs.,2000,7:301-311
[9]Sheldrick G M.SADABS,Program for Empirical Absorption Correction of Area Detector Data,University of G?ttingen, Germany,1996.
[10]Sheldrick G M.SHELXS 97,Program for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
[11]Le Pecq JB,PaoletliC.Anal.Biochem.,1996,17:100-107
[12]Efthimiadou E K,Psomas G,Sanakis Y,et al.J.Inorg. Biochem.,2007,101:525-535
[13]CarterM T,Allen J.J.Am.Chem.Soc.,1987,109:7528-7530
[14]Hertzberg R P,Dervan P B.J.Am.Chem.Soc.,1982,104: 313-315
[15]PU Xiao-Hua(蒲小華),YANG Pin(楊頻).Chinese J.Inorg. Chem.(WujiHuaxue Xuebao),2010,26(9):1567-1572
[16]Zhou J,Wang L F,Wang J Y,et al.J.Inorg.Biochem., 2001,83:41-48
[17]ZHOU Qing-Hua(周慶華),YANG Pin(楊頻).Acta Chim Sin.(Huaxue Xuebao),2006,64(8):95-100
Two Rare Earth Com plexes Based on Enoxacin:Synthesis, Crystal Structures,DNA Binding and Antibacterial Activities
DENG Ji-Hua MEIGuang-Quan*
(College of Chemistry and Bio-engineering,Key Laboratory of JiangxiUniversity for Applied Chemistry and Chemical Biology,Yichun University,Yichun,Jiangxi 336000,China)
Two rare earth complexes based on enoxacin(HEx),[Ce(Ex)4]·39H2O(1)and[Sm2(Ex)4(HEx)2(ox)]· 24H2O(2)(HEx=enoxacin;ox=oxalate),have been synthesized and characterized by IR,EA and X-ray diffraction. The analysesof the single X-ray diffraction results indicate that1 isamononuclear Ce髧complex and crystallizes in tetragonal system,space group I41/a with a=1.551 74(12)nm,c=4.066 6(4)nm,V=9.791 9(14)nm3,Z=4,μ= 0.574mm-1,F(000)=4 480,Dc=1.441 g·cm-3,R1=0.074 3,wR2=0.180 0;2 is an oxalate-bridged binuclear Sm(Ⅲ)complex and crystallizes in triclinic system,space group P1 with a=1.505 72(19)nm,b=1.511 98(10)nm,c= 1.610 58(10)nm,α=116.723(2)°,β=107.987(2)°,γ=98.020(2)°,V=2.943 0(5)nm3,Z=1,μ=1.095 mm-1,F(000)= 1 412,Dc=1.545 g·cm-3,R1=0.039 4,wR2=0.121 7.The bindings of 1 and 2 with calf thymus DNA(CT-DNA) have been investigated by electronic absorption spectroscopy and emission spectroscopy.The results indicate that both complexes bind with double-stranded CT-DNA mostly via intercalative mode,with the intrinsic binding constants(Kb)of4.45×104and 6.56×104mol-1·L,respectively.Antibacterial tests show both complexeshave better antibacterialactivitiesagainstGram and fungimicrobial than the free ligand HEx.CCDC:751128,1;751129,2.
enoxacin;rare earth complex;crystal structure;DNA binding;antibacterial activity
book=2463,ebook=27
O614.33+2;O614.33+7
A
1001-4861(2012)11-2431-06
2011-12-06。收修改稿日期:2012-06-12。
江西省自然(青年)科學基金(No.2010GZH0126),江西省教育廳科技計劃(No.GJJ09637,GJJ12577,GJJ11603,GJJ10701)資助項目。
*通訊聯系人。E-mail:djhycu_2006@yahoo.com.cn,yc_mgq@163.com

