組合型Pt3Sn/Al2O3催化劑用于芳香硝基化合物一鍋法合成N-烷基芳胺
楊 芳 許響生 顧輝子 陳傲昂 嚴新煥
(浙江工業大學綠色化學合成技術國家重點實驗室培育基地,杭州310014)
1 Introduction
N-alkyl anilines are widely used as synthetic intermediates for pharmaceuticals,agrochemicals,fine chemicals,bioactive compounds,and dye chemicals.1,2The most commonly used method for N-alkylation is the coupling of amines with alkyl halides.3-5This procedure,however,can be problematic because of the toxic nature of many alkyl halides,as well as the concomitant formation of large quantities of undesired waste. The reductive amination of aldehydes and ketones is another well-known method.6-8Unfortunately,this method requires the usage of strong reducing reagents.In comparison with the methodologies mentioned above,transition metal-catalyzed amine alkylation with alcohols has attracted much interest thanking to the ubiquitous availability of alcohols,high atom efficiency,and formation of water as the sole byproduct.In the former reports,extensive attention has been focused on Pd,9,10Ru,11,12Au,13Ir,14,15Ni,16and Zn17,18as effective catalysts.But,anilines need to be firstly synthesized by the reduction of nitroaromatics.Therefore,direct synthesis of N-alkyl anilines from nitroaromatics and alcohols as starting materials in one-pot reactions is an important issue in chemistry owing to several inherent advantages such as simplifying separation steps,reducing the use of reagents,and increasing yields.Recently,it was reported that N-alkyl anilines could be synthesized in one-pot using nitroaromatics as starting materials with alcohol as alkylation agent.19-24But,most of the reports focused on homogeneous catalytic systems,which are not practically useful due to the problem of wax-catalyst separation,the indispensable use of large amounts of addictives or co-catalysts, highly demanding scaling-up,and catalystdeactivation.25Therefore,it would be highly desirable if a heterogeneous catalyst could be used in one-pot synthesis of N-alkyl anilines from nitroarenes without additional hydrogen gas and organic ligand or base,simplifying separation steps,enhancing the life time of the catalyst.
In this study,we report one-pot synthesis of N-alkyl anilines from nitroaromatic catalyzed by a simple and versatile PtxSn/ Al2O3(molar ratio of Pt and Sn is x:1)heterogeneous catalyst in the presence of water in a continuous fixed-bed reactor (Scheme 1).
2 Experimental
2.1 Catalyst preparation
A γ-alumina(i.d.,2-3 mm,surface area,238 m2·g-1,Zhejiang jingjing alumina Ltd.)was used as support,which was previously calcined in flowing air at 773 K for 5 h.The assembled PtxSn/Al2O3catalyst was prepared by the method of absorption as following.(1)the γ-alumina was impregnated with an appropriate concentration of SnCl2·2H2O(CP,Shanghai Experimental Reagent Ltd.)to get a precursor.The precursor was stirred for 1 h and kept for 24 h,then the excess solvent was removed by heating at 333 K.Then the precursor was calcined in air at 623 K for 3 h,signed as Sn/Al2O3.(2)The procedure to prepare Pt2(dba)3(dba=dibenzylideneacetone)was described in the literature.26,27Pt2(dba)3(34 mg)in 200 mL of propylene carbonate was placed in a 500 mL stainless autoclave.And the system purged three times with hydrogen.Hydrogenation was carried out under 4.0 MPa hydrogen pressure at ambient temperature for about 2 h under vigorous stirring to obtain brown Pt precursor.(3)For depositing the sol on the support,the obtained Sn/Al2O3was added to Pt precursor solution under stirring for 24 h,and then the solid catalyst was separated by filtration and purification by acetone to get PtxSn/Al2O3catalyst. The actual metal content of the catalyst was determined by Shimadzu inductively coupled plasma mass spectrometry (ICP-MS)measurements.
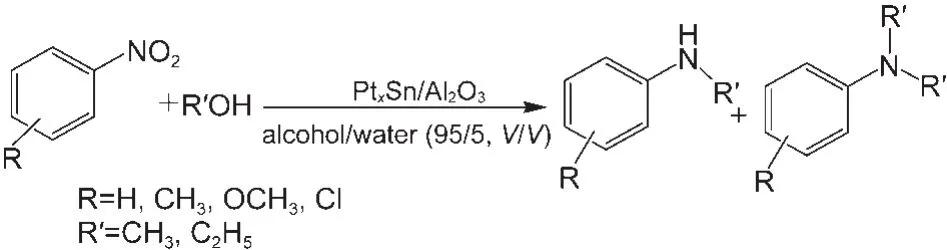
Scheme 1 Synthesis of N-alkyl aniline
2.2 Catalyst characterization
The Brunauer-Emmett-Teller(BET)specific surface area was measured using nitrogen volumetric adsorption(BET,Micromeritics ASAP 2010)at 77 K.Prior to measurement,the samples were degassed to 0.1 Pa at 373 K.The specific surface areas were calculated in a relative pressure range(0.05
Transmission electron microcopy(TEM)photographs were taken by using a JEOL JEM-200C electron microscope.The catalyst powders were dispersed in ethanol by ultrasonic vibration.One drop of the solution was then deposited onto a thin holey-carbon film supported on lacey-carbon/Cu grid(300 Mesh)and left to dry.The powder grains based the metal particles were well separated and supported on the thin holey-carbon film and then characterized by TEM.TEM was operated at an accelerating voltage of 200 kV.
X-ray photoelectron spectroscopy(XPS)was acquired with ESCALab220i-XL.A resolution of 0.1 eV was used to determine the metal atomic ratio of the surface region and the metal oxidation state of the selected catalysts.Samples were in powder form and were pressed on a double-side adhesive copper tape.All measurements were carried out at room temperature without any sample pre-treatment.An Al KαX-ray source was used in this work.To compensate for surface charge effects binding energies(EB)were calibrated using C 1s hydrocarbon peak at 284.6 eV.
2.3 Catalytic reactions
The catalytic activity of the PtxSn/Al2O3was tested by the hydrogenation and further N-alkylation of nitrobenzene.The catalytic tests were performed in a continuous fixed-bed reactor designed by our group.28The catalyst(4 g)was loaded in the isothermal region of the reactor.The reaction temperature was kept constant by CHB 702 thermometer within±0.1°C,and the corresponding pressure was controlled by GO BP-60 Back Pressure control regulator.The appropriate pressure was to ensure that the reactant was in liquid phase.The corresponding concentration of nitrobenzene was dissolved in ethanol/water solution.A HPLC pump(Model 501style,Syltech Corporation)was adopted to pump the reactant into the fixed-bed reactor with internal diameter of 0.52 cm at liquid hourly space velocity(LHSV)of 7.5 h-1.After reacting in the fixed-bed reactor,the liquid and gas were separated in the vapor-liquid separator.The reaction products were identified by GC-MS(Agilent-6890 GC-5973 MS equipped with 30 m HP-5 capillary), and quantified by GC(GC-9790 equipped with a flame ioniza-tion detector and SE-30 capillary column)every 2 h.The oven temperature of both was 453 K,injector temperature was 523 K,and detector temperature was 533 K.
3 Results and discussion
3.1 Catalyst characterization
The BET specific surface area of the Pt3Sn/Al2O3is 222.4 m2·g-1,and the corresponding pore volume and pore diameter are 0.5 cm3·g-1and 8.6 nm,respectively.The TEM micrograph of the fresh catalyst is shown in Fig.1a.It is obvious that the Pt particles are highly dispersed on γ-alumina,and the mean size of Pt particles is 1.9 nm(Fig.1b).
XPS data are shown in Fig.2.No indication can be drawn regarding alloy formation on our samples from XPS data due to limitations of this technique.Because the energy region of Pt 4f levels was overshadowed by the presence of a very strong Al 2p peak,29,30thus Pt3Sn/C was analyzed instead.In this region,the spectra show two peaks.The one at lower binding energy corresponds to Pt 4f7/2level and at the higher energy to the Pt 4f5/2.The binding energy of 71.0 eV can be ascribed to Pt(0), and the binding energy of 72.6 eV can be assigned to the presence of Pt(II)species.The Sn 3d spectra of the Pt3Sn/C catalyst show two peaks at about 488.2 and 485.3 eV.On the basis of literature indication,31the peak of lower intensity at 485.3 eV can be characteristic of tin in metallic whereas that at 488.2 eV can be assigned to the oxidized tin species(Sn(II)and/or Sn(IV)).Unfortunately,distinguishing oxide forms of tin is difficult because their binding energies are too close.The Pt 4f7/2and Sn 3d5/2at binding energies of 71.0 and 485.3 eV,respectively are characteristic of Pt and Sn atoms in the Pt3Sn alloy, which is agreement with the results of Dupont et al.32
3.2 Catalytic performance of different Pt-based catalysts
Firstly,the catalytic activity and selectivity for the N-alkylation of nitrobenzene to the corresponding N-alkyl anilines were compared among various catalysts(Table 1).As for entry 1, 45.9%aniline was observed if using Pt/Al2O3as the catalyst although 58.8%nitrobenzene was reduced.In entry 2,the introduction of tin could remarkably improve the formation of N-alkyl aniline,i.e.,the conversion of aniline to N-alkyl aniline. With the presence of Sn,Sn would dilute Pt sites and increase the electron density of Pt.When the molar ratio of Pt and Sn alters from 5 to 3,the conversion of nitrobenzene increases from 99.0%to 100%,and the total yields of N-ethyl aniline and N, N-diethyl aniline increase from 70.0%to 98.2%(entries 2-4). The conversion is 100%and the selectivity to N-alkyl anilines is 98.2%using 1%Pt3Sn/Al2O3catalyst(the mass ratio of Pt and Al2O3is 1%)(entry 4).When the molar ratio of Pt and Sn is 2(entry 6),the conversion does not change,but the selectivity of N-alkyl anilines decreases compared to the 1%Pt3Sn/ Al2O3.This could be explained from the results of XPS.When the molar ratio of Pt and Sn is 3:1,the metallic Pt and Sn would exist in the formation of Pt3Sn alloy,which would be favorable for hydrogenation of N=O.We also investigate the ef-fect of the loading of Pt increasing from 0.5%to 2%,the results show that there is only little distinction on the conversion and selectivity(entries 4,6,7).This illustrates that 0.5%Pt has sufficient active sites in the catalyst.However,0.5%Pt is easily leaching with the reaction.
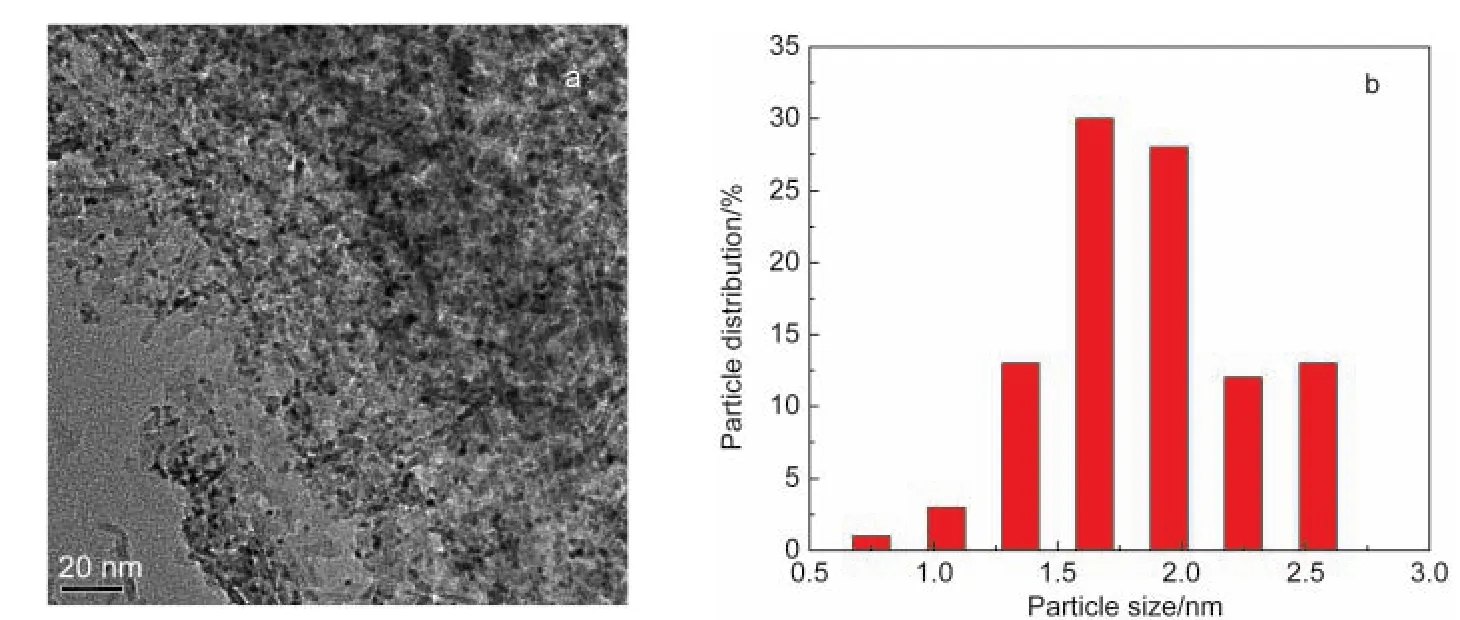
Fig.1 (a)TEM image of fresh Pt3Sn/Al2O3and(b)size distribution of Pt particles on the fresh catalyst
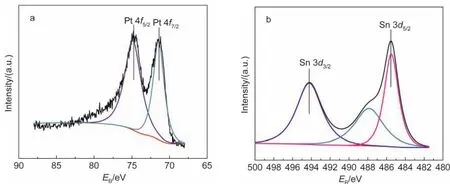
Fig.2 XPS spectra of(a)Pt 4f and(b)Sn 3d of the fresh Pt3Sn/C catalyst
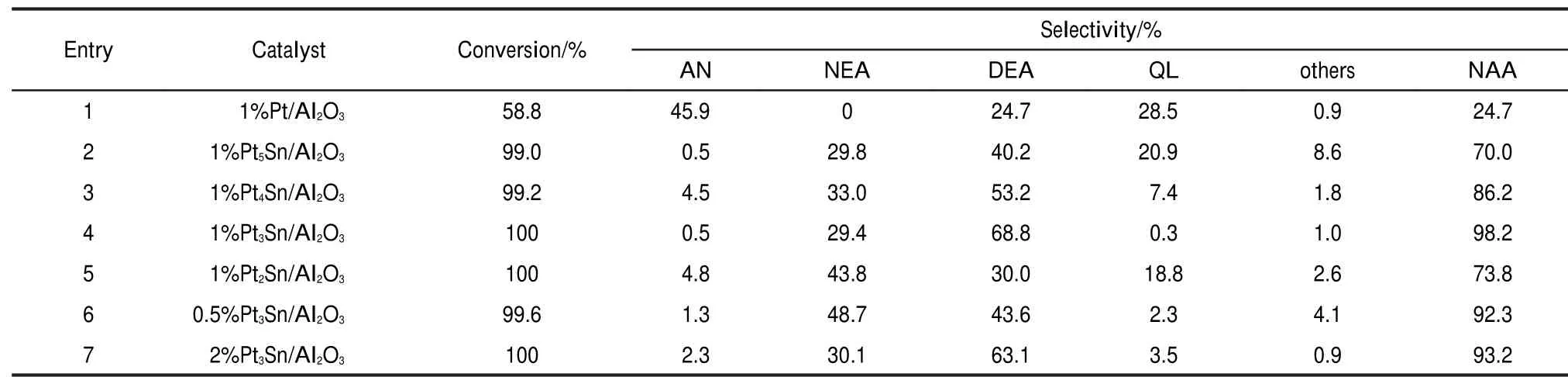
Table 1 Results of different catalysts on nitrobenzene
3.3 Effect of reaction conditions of the N-alkylation of nitrobenzene
The effects of the reaction conditions on the conversion and yield are gathered in Table 2.In the reaction,water plays an important role,therefore,confirming that the optimum percentage of water is necessary.The effect of water on the reaction is shown in entries 8-11.When the volume ratio of ethanol/water increases from 70/30 to 95/5,no obvious changes on conversion are observed,but the yield of the N-alkyl anilines increases significantly.Besides,the yield of N,N-diethyl aniline also increases with the increasing of the volume ratio of ethanol/water,while that of the quinaldine decreases.This could be explained that with increasing water volume,the efficient of steam reforming would be higher.28However,in the reaction, higher active hydrogen(H*)production is unfavourable to the yield of aniline and further N-alkylation to corresponding N-alkyl aniline because it would cause over-hydrogenation.In addition,reaction temperature would affect catalytic activity and selectivity significantly.It is obvious that with increasing the reaction temperature from 443 to 503 K,the conversion of nitrobenzene raises from 81.2%to 100%,and the yield of the total N-alkyl aniline raises from 80.9%to 98.2%(entries 11-14). The result suggests that higher reaction temperature produces higher N-alkyliniline,whereas it would produce higher quantities of C-alkylated products if the reaction temperature is higher according to previously reported results.33Besides,the concentration and LHSV of nitrobenzene are also investigated(entries 15-20).With the increase of the concentration and LHSV of nitrobenzene,the conversion and the yield decrease.This could be explained by the following:with the increase of the concentration and LHSV of nitrobenzene,the contact time between the reactant and the catalyst decreases.
3.4 N-alkylation of nitroaromatics with alcohols to the corresponding N-alkyl anilines
Then the scope of the reaction with respect to nitrobenzene with aliphatic alcohols was investigated(Table 3).Methanol and nitrobenzene proceeded efficiently with 85.5%yield(entry 21).When using ethanol as alkylation agent,the yields of N-alkyl anilines are up to 98.2%(entry 22).Compared to metha-nol,ethanol used as alkylated agent gives the higher yields of target compounds,especially higher conversion of anilines, thanking to high hydrogen atom utilization.Isopropanol,butanol,and cyclohexanol used as alkylated reagents react smoothly with nitrobenzene,giving the corresponding product in moderate yields(entries 23-25),and higher selectivity of di-alkyl aniline than mono-alkyl aniline.This could be attributed to the steric effect.However,the benzyl alcohols do not react due tothe poor solubility.This indicates that hydrogen produced by aliphatic alcohol/water reforming is feasible.
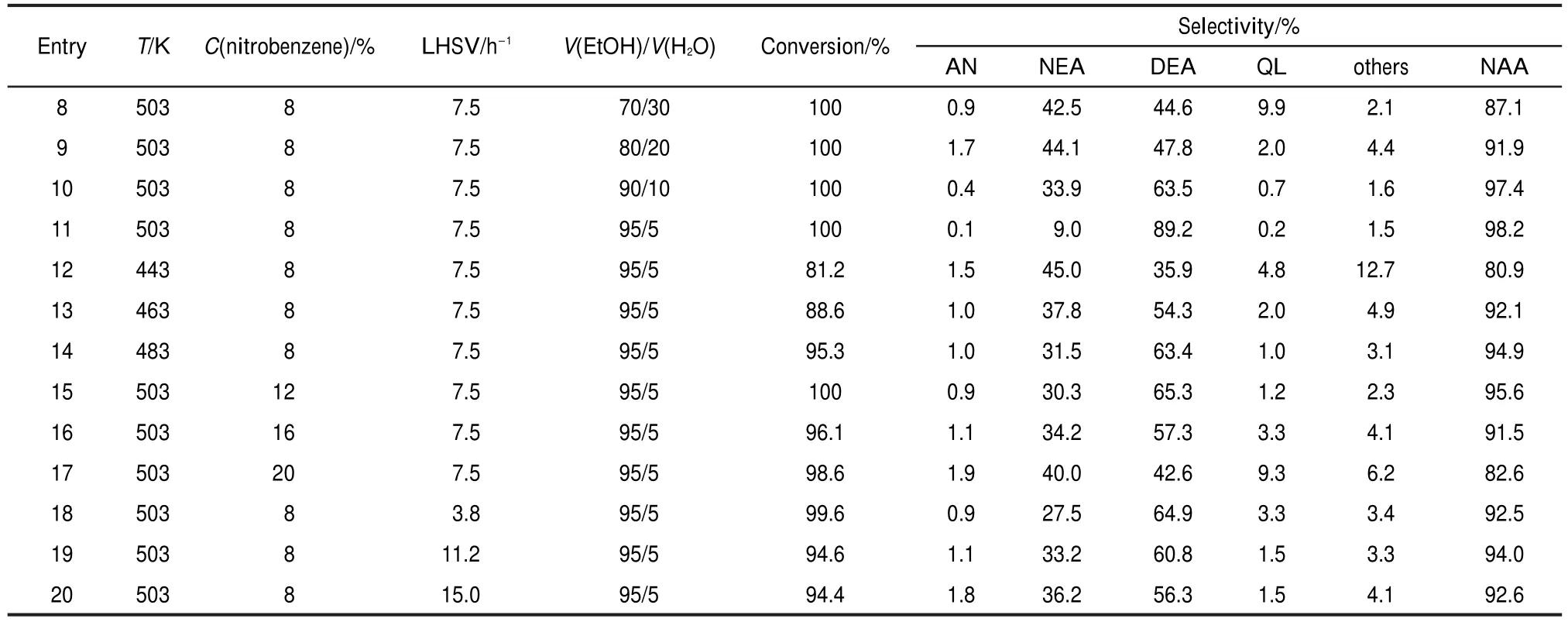
Table 2 Effect of reaction conditions on hydrogenation and N-alkyl aniline of nitrobenzene
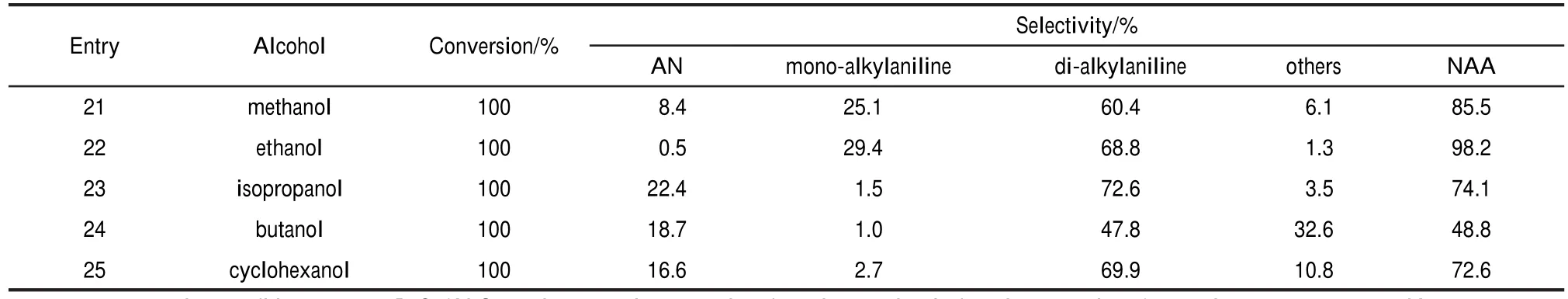
Table 3 Alkylation of nitrobenzene with different aliphatic alcohols

Table 4 Alkylation of different nitroaromatics with ethanol
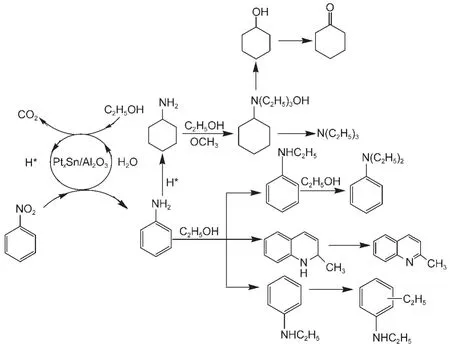
Fig.3 Possible mechanism of one-pot synthesis of N-ethyl anilne and N,N-diethyl aniline from nitrobenzene
The results for the alkylation of nitroarenes with ethanol are shown in Table 4.With 1%Pt3Sn/Al2O3as a catalyst,the ethylation of different nitroarenes containing electron-donating substituents in ethanol goes smoothly and produces the corresponding N-ethyl and N,N-diethyl anilines,more than 90% yields are obtained(entries 26-32).This could be explained by the electronic effect and synergetic effect of Sn,which would decrease the electronic density of N atom.The electron abundant Pt atom would absorb the electron deficienct N atom, strengthening the adsorption of reactant,which is benefit for the hydrogenation reaction.However,the substituents at different positions on the nitroarenes affect the reaction yield slightly(entries 27-29).The yield of para position is better than that of ortho position,which could be explained by the stereoscopic effect.The reaction tolerates the presence of substituents(entries 33-37).Meanwhile,the yields of products decrease slightly due to the electron-withdraw group,which agrees with the result pointed out by Kawahara et al.14Interestingly,two halogenated atoms presented in the substrate exhibit high selectivity of N-alkyl aniline with the yield larger than 94%(entries 38,39).
3.5 Possible mechanism of in situ hydrogenation N-alkylation
Although the mechanism for the present reaction is not completely clear yet,a possible mechanism is shown in Fig.3.Firstly,the nitro group is reduced to amine by H*produced by ethanol/water reforming.And ethanol dehydrogenates to corresponding acetaldehyde.In the next step,two different routes are proposed.On the one hand,the amine reacts with the alcohol to give N-ethyl aniline.The produced N-ethyl aniline further undergoes substitution of N-ethylation with ethanol as alkylation reagent,getting N,N-diethyl aniline.On the other hand,aldol condensation would happen between ethanol and acetaldehyde,and further react with amine to 2-methyl-quinoline.Besides,the amine reacts with H*to corresponding cyclohexanamine,therefore,rich H*and water conditions should be avoid.
4 Conclusions
In summary,the one-pot synthesis of N-alkyl anilines from nitroaromatics was successfully realized with assembled Pt3Sn/ Al2O3as catalyst in a continuous fixed-bed reactor.The conversion of nitrobenzene was 100%,with the total yield of N-ethyl and N,N-diethyl anilines of 98.2%at the optimum reaction conditions(503 K,7.5 h-1,5%water).This novel reaction contains the advantages such as highly hydrogen atom utilization,simplification procedure,and environment-friendly.
(1) Xia,J.H.;Zhang,X.;Matyjaszewski,K.ACS Symp.Ser.2000, 760,207.doi:10.1021/bk-2000-0760.ch013
(2) Clardy,J.M.;Fischbach,A.;Walsh,C.T.Nat.Biotechnol. 2006,24,1541.doi:10.1038/nbt1266
(3) Oku,T.;Arita,Y.;Tsuneki,H.;Ikariya,T.J.Am.Chem.Soc. 2004,126,7368.doi:10.1021/ja048557s
(4) Salvatore,R.N.;Nagle,A.S.;Jung,K.W.J.Org.Chem.2002, 67,674.doi:10.1021/jo010643c
(5) Basu,B.;Paul,S.;Nanda,A.K.Green Chem.2009,11,1115. doi:10.1039/b905878h
(6) Tripathi,R.P.;Verma,S.S.;Pandey,J.;Tiwari,V.K.Curr.Org. Chem.2008,10,1093.
(7) Byun,E.;Hong,B.;De Castro,K.A.;Lim,M.;Rhee,H.J.Org. Chem.2007,72,9815.doi:10.1021/jo701503q
(8)Abdel-Magid,A.F.;Mehrman,S.J.Org.Process Res.Dev. 2006,10,971.doi:10.1021/op0601013
(9)Shimizu,K.;Shimura,K.;Ohshima,K.;Tamura,M.;Satsuma, A.Green Chem.2011,13,3096.doi:10.1039/c1gc15835j
(10)Xu,C.;Xiao,Z.;Zhou,B.;Wang,Y.;Huang,P.Chem. Commun.2010,46,7834.doi:10.1039/c0cc01487g
(11)Yamaguchi,K.;He,J.;Oishi,T.;Mizuno,N.Chem.Eur.J. 2010,16,7199.
(12)Hamid,M.H.S.;Allen,A.C.L.;Lamb,G.W.;Maxwell,A.C.; Maytum,H.C.;Waston,A.J.A.;Williams,J.M.J.J.Am. Chem.Soc.2009,131,1766.doi:10.1021/ja807323a
(13)He,L.;Lou,X.;Ni,J.;Liu,Y.;Cao,Y.;He,H.;Fan,K.Chem. Eur.J.2010,16,13965.doi:10.1002/chem.201001848
(14) Kawahara,R.;Fujita,K.;Yamaguchi,R.Adv.Synth.Catal. 2011,353,1161.doi:10.1002/adsc.201000962
(15) Saidi,O.;Blacker,A.J.;Farah,M.M.;Marsden,S.P.; Williams,J.M.J.Chem.Commun.2010,46,1541.doi: 10.1039/b923083a
(16) Ruano,J.L.G.;Parra,A.;Aleman,J.;Yuste,F.;Mastranzo,V. M.Chem.Commun.2009,404.
(17) Zhu,A.;Li,L.;Wang,J.;Zhuo,K.Green Chem.2011,13,1244. doi:10.1039/c0gc00763c
(18) Enthaler,S.Catal.Lett.2011,141,55.doi:10.1007/ s10562-010-0463-4
(19)Peng,Q.;Zhang,Y.;Shi,F.;Deng,Y.Q.Chem.Commun.2011, 47,6476.doi:10.1039/c1cc11057h
(20) Pandarus,V.;Ciriminna,R.;Beland,F.;Pagliaro,M.Catal.Sci. Technol.2011,1,1616.doi:10.1039/c1cy00097g
(21)Lee,C.C.;Liu,S.T.Chem.Commun.2011,47,6981.doi: 10.1039/c1cc11609f
(22)Xiang,Y.Z.;Li,X.N.;Lu,C.;Ma,L.;Zhang,Q.Alpplied Catal.A:Gen.2010,375,289.doi:10.1016/j. apcata.2010.01.004
(23) Feng,C.;Liu,Y.;Peng,S.;Shuai,Q.;Deng,G.;Li,C.Org.Lett. 2010,12,4888.doi:10.1021/ol1020527
(24)Bea,J.W.;Cho,Y.J.;Lee,S.H.;Yoon,C.M.;Yoon,C.M. Chem.Commun.2000,1857.
(25) Reddy,R.C.;Vijeender,K.,Bhusan,B.P.;Madhavi,P.P.; Chandrasekhar,S.Tetrahedron Lett.2007,48,2765.doi: 10.1016/j.tetlet.2007.02.050
(26) Ely,T.O.;Pan,C.;Amiens,C.;Chaudret,B.;Dassenoy,F.; Lecante,P.;Casanove,M.J.;Mosset,A.;Respaud,M.;Broto,J. M.J.Phys.Chem.B 2000,104,695.doi:10.1021/jp9924427
(27) Mao,J.Z.;Yan,X.H.;Gu,H.Z.;Jiang,L.C.Chin.J.Catal. 2009,30,182.[毛建忠,嚴新煥,顧輝子,江玲超.催化學報, 2009,30,182.]doi:10.1016/S1872-2067(08)60095-9
(28) Zhou,L.;Gu,H.Z.;Yan,X.H.Catal.Lett.2009,132,16.doi: 10.1007/s10562-009-0036-6
(29)Riguetto,B.A.;Damyanova,S.;Goluliev,G.;Marques,C.M. P.;Petrov,L.;Bueno,J.M.C.J.Phys.Chem.B 2004,108,5349.
(30) Navarro,R.M.;álvarez-Galván,M.C.;Sánchez-Sánchez,M. C.;Rosa,F.;Fierro,J.L.G.Appl.Catal.B:Environ.2005,55, 229.doi:10.1016/j.apcatb.2004.09.002
(31) Merlen,E.;Beccat,P.;Bertolini,J.C.;Delichère,P.;Zanier,N.; Didillon,B.J.Catal.1996,159,178.doi:10.1006/ jcat.1996.0077
(32) Dupont,C.;Delbecq,F.;Loffreda,D.;Jugnet,Y.J.Catal.2011, 278,239.doi:10.1016/j.jcat.2010.12.012
(33) Narayanan,S.;Deshpande,K.Appl.Catal.A 1996,135,125. doi:10.1016/0926-860X(95)00220-0