海洋鏈霉菌S007次生代謝產(chǎn)物的分離鑒定
張宏宇,秦 梅,李富超,LAATSCH Hartmut,王宏鵬,秦 松
1中國科學(xué)院煙臺海岸帶研究所,煙臺264003;2中國科學(xué)院研究生院,北京100049; 3曲阜師范大學(xué)化學(xué)與化工學(xué)院,曲阜273165;4中國科學(xué)院海洋研究所,青島266071; 5Department of Organic and Biomolecular Chemistry,University of G?ttingen,G?ttingen,D-37077,Germany
The ocean is a diverse assemblage of life forms.Marine microorganisms live in the environment of extreme variations in pressure,salinity,and temperature.They have developed unique metabolic and physiological capabilities to offer the potential for the production of special metabolites that can ensure them to survival,this cannot be observed from terrestrial microorganisms[1].Our group has done the research on marine streptomycete for novel natural products with potential antitumor activity for 12 years[2-6].As part of this work,the crude extract of Streptomyces sp.S007 from sediments of Lianyungang showed significant toxicity against the brine shrimp was studied.As a result,nine compounds were isolated.All the compounds were evaluated for cytotoxic effects on the brine shrimps,only compound 9 showed significant toxicity.This strain may be a potential streptomycete to produce kalamycin.
Materials and Methods
Materials
Marine Streptomyces sp.S007 was obtained from the marine sediments in Lianyungang,Jiangsu Province. Brine shrimp was bought in Germany.Gause’s synthetic agar(for separation of streptomycete):soluble starch 20 g,KNO31 g,K2HPO40.5 g,MgSO4·7H2O 0.5 g,F(xiàn)eSO4·7H2O 0.01 g,K2Cr2O70.3 g,seawater 500 mL,deionized water 500 mL,pH 7.4.M2+medium (for fermentation):malt extract 10 g,yeast extract 4 g,anhydrous glucose 4 g,deionized water 500 mL,seawater 500 mL,pH 7.8.NMR spectra were recorded on Mercury-300 spectrometers and TMS was used as internal standard.El-MS spectra were recorded on a VG Autospec-3000 spectrometer.ESI-MS spectra on Quattro Triple Quadruple Finigan MAT-In-cos50.Column chromatography was carried out on silica gel(200-300 mesh)and Sephadex LH-20(Amersham Biosciences,Uppsala,Sweden).All reagents are analytically pure. Instruments and reagents
NMR spectra were recorded on Mercury-300 spectrometers and TMS was used as internal standard.El-MS spectra were recorded on a VG Autospec-3000 spectrometer.ESI-MS spectra on Quattro Triple Quadruple Finigan MAT-In-cos50.Column chromatography was carried out on silica gel(200-300 mesh)and Sephadex LH-20(Amersham Biosciences,Uppsala,Sweden).All reagents are analytically pure.
Fermentation and extraction
The strain Streptomycete sp.S007 was inoculated in the solid M2+medium in the culture plate.After 3 days,when it grew well,it was inoculated into liquid M2+ medium to carry out the fermentation.Fermentation was carried out in 1000 mL flasks containing 300 mL medium on a rotary shaker at 150 rpm,28℃ for 4 days. The culture broth(30 L)S007 was filtered and the mycelium and water phase were obtained.The mycelium was extracted with ultrasonic extraction by ethyl acetate and acetone successively for three times,15 minutes per time,and checked by TLC.The water phase was loaded in the resin column XAD-16,washed slowly with water and methanol successively.Methanol phase was collected and distilled under reduced pressure.After this,aqueous solution of the crude extract was given.This aqueous solution was extracted with ethyl acetate and checked with TLC.
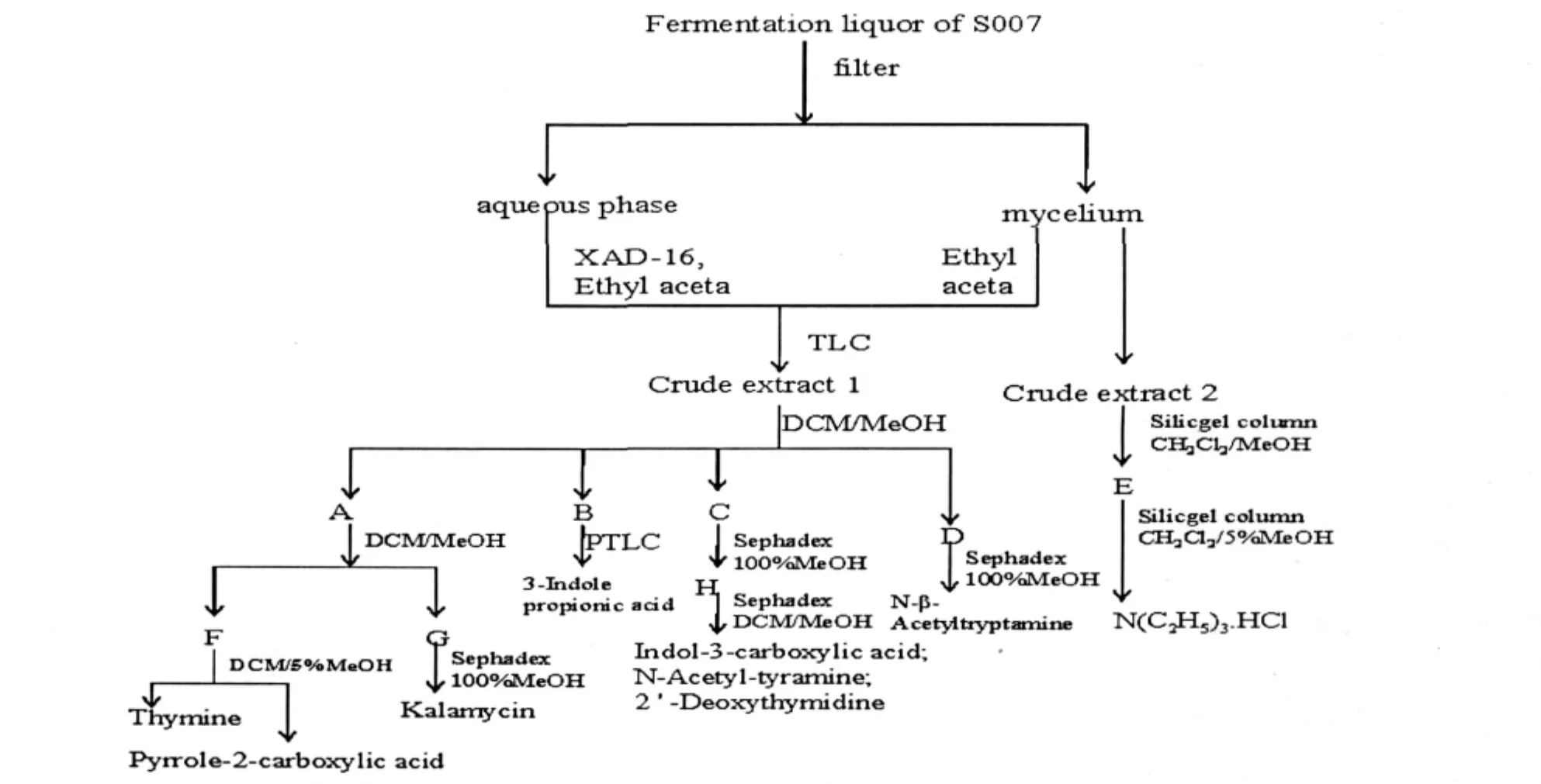
Fig.1 The separation procedure of nine compounds–
Bioassay and Isolation of the crude extract
The brine shrimp bioassay of the crude extract showed marked toxicity to brine shrimp larvae at the concentra tion of 1 mg/mL,with mortality rate of 78.5%(from water phase).Silica gel column chromatography was utilized to separate the crude extract,the solvent is CH2Cl2/(1%,3%,5%,7%,10%,20%,50%) MeOH,five fractions were obtained.Fraction E(2.82 g)was separated over silica gel column(CH2Cl2/5% MeOH)to give C6H15N·HCl(9,100.3 mg).Fraction D(0.67 g)was separated over Sephadex LH-20 column to give N-β-acetyltryptamine(8,20.7 mg). The repeated separation of Fraction A(2.62 g)over silica gel column and Sephadex LH-20 afford thymine (1,97.4 mg),kalamycin(3,8.4 mg),and pyrrole-2-carboxylic acid(2,22.6 mg).Fraction C(2.31 g) was separated over Sephadex LH-20 for two times to afford indol-3-carboxylic acid(5,24.4 mg),N-acetyl-tyramine(6,139.2 mg)and 2'-deoxythymidine(7,6.1 mg).3-Indole propionic acid(4,6.3 mg)was obtained from separation of Fraction B(1.25 g)over PTLC.
Bioassay of the nine compounds[7-8]
Nine compounds at the concentration of 40 μg/mL were submitted to brine shrimp(Artemia salina)bioassay for toxicity.
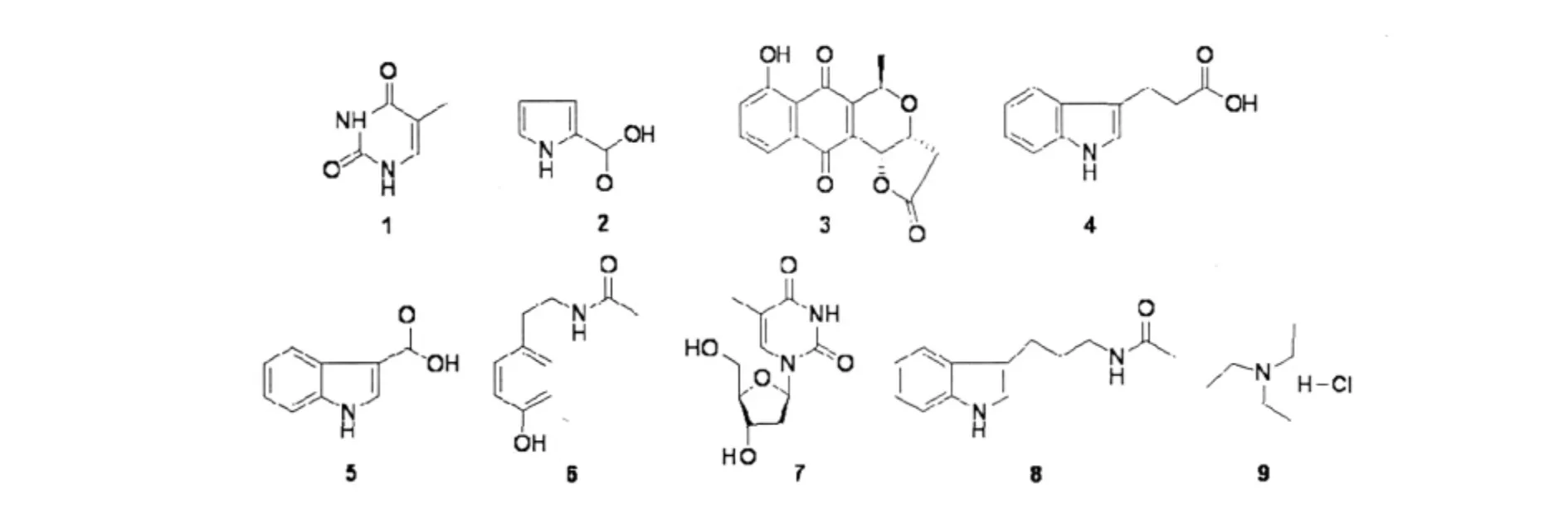
Fig.2 Nine compounds from the fermented broth of Streptomycete sp.S007
Identification
Thymine (1) White needle-shaped crystal (CH2Cl2),C5H6N2O2,Rf= 0.34(CH2Cl2/5% MeOH).1H NMR(DMSO-d6,300 MHz)δ:10.6-10.9 (m,H-3,H-1),7.23(d,J=0.8 Hz,H-6),1.73(d,J =0.6 Hz,5-CH3).The above spectral data were in agree with the reported data of thymine in the Antibase.[9]
Pyrrole-2-carboxylic acid(2)White needle-shaped crystal(CH2Cl2),C5H5NO2,Rf=0.51(CH2Cl2/5% MeOH).1H NMR(CD3OD,300 MHz)δ:6.94-6.92 (m,H-2),6.86-6.84(m,H-4),6.18-6.17(m,H-3).13C NMR(CD3OD,125 MHz)δ:164.9(s,C-6),124.4(s,C-2),123.7(s,C-5),116.6(s,C-4),110.5 (s,C-3).HR-ESI-MS(m/z:134.0[M+Na]+,calcd.for(C5H5NO2,111.0987).According to checking the data of1H NMR in AntiBase,compound 2 is pyrrole-2-carboxylic acid.These evidence above were in agreement with the literature values of pyrrole-2-carboxylic acid.[10]
Kalamycin(3)Yellow solid,C16H12O6,Rf=0.65 (CH2Cl2/5%MeOH),1H NMR(CDCl3,300 MHz) δ:11.8(s,9-OH),7.30(dd,7.5,2.1 Hz,H-8),7.73-7.65(overlapping,H-6,7),5.27(d,3.0 Hz,H-4),5.09(q,6.9 Hz,H-1),4.70(dd,5.1,3.0 Hz,H-5),2.97(dd,17.7,5.2 Hz,H-13β),2.71(d,17.7,H-13α),1.57(d,6.9 Hz,1-CH3).According to checking the data of1H NMR in AntiBase,compound 3 is kalamycin.[9]
3-Indolepropionic acid (4) White crystal (CH2Cl2),C11H11NO2,Rf= 0.59(H2Cl2/5% MeOH),1H NMR(CD3OD,300 MHz)δ:8.18-8.21 (m,H-3),7.74(m,H-9),7.32-7.35(m,H-5,H-6),7.03-7.11(m,H-4),3.31-3.28(m,10-CH2),2.36-2.41(m,11-CH2).According to checking the data of1H NMR in AntiBase,compound 4 is 3-indole propi-onic acid.[9]
Indol-3-carboxylic acid (5) White crystal (CH2Cl2),C9H7NO2,Rf=0.58(CH2Cl2/5%MeOH) .1H NMR(CD3OD,300 MHz)δ:8.06(dd,J=6.0,2.5 Hz,H-6),7.93(s,H-9),7.45-7.40(m,H-4),7.18(m,H-5),7.06-7.11(m,H-3).According to checking the data of1H NMR in AntiBase,compound 5 is indol-3-carboxylic acid.[9]
N-acetyl-tyramine(6)White powder,C10H13NO2,Rf=0.39(CH2Cl2/10%MeOH).1H NMR(CD3OD,300 MHz)δ:7.07-6.96(m,H-5,H-3),6.76-6.67 (m,H-2,H-6),3.31(m,9-CH2),2.66(m,8-CH2),1.89(s,13-CH3).13C NMR(CD3OD,125 MHz)δ: 172.97(s,C-9),156.7(s,C-1),131.1(s,C-4),130.4(s,C-3,C-5),116.2(s,C-2,C-6),42.3(s,C-8),35.6(s,C-7),22.6(s,C-10).According to checking the data of1H NMR in AntiBase,compound 6 is N-acetyl-tyramine.The above spectral data were agree with the literature valuas of N-acetyl-tyramine.[9]
2’-deoxythymidine(7) White crystal(CH2Cl2/ 10%MeOH),C10H14N2O5,Rf=0.58(CH2Cl2/5% MeOH).1H NMR(CD3OD,300 MHz)δ:7.82-7.78 (m,H-11),6.29(d,J=6.98 Hz,H-2),3.89(dd,J =6.7,3.3 Hz,H-4),3.80-3.67(m,H-5),3.40-3.23(m,13-CH2),2.22(ddd,J=7.8,5.2,3.6 Hz,3-CH2),1.88(dd,J=6.22,2.29 Hz,17-CH3) .According to checking the data of1H NMR in Anti-Base,compound 7 is 2'-deoxythymidine.The above spectral data were in agree with the literature values of 2'-deoxythymidine.[11]
N-β-acetyltryptamine(8) C13H16N2O,Rf=0.30 (CHCl3/10%MeOH);1H NMR(CD3OD,300 MHz) δ:7.55(dt,H-4),7.32(dt,H-7),7.05(ddd,H-6),6.99(ddd,H-5),3.45(t,H-2'),2.93(t,H-1'),1.90(s,CH3).Compound 8 is N-β-acetyltryptamine.The above spectral data were in agree with the literature values of N-β-acetyltryptamine.[12]
Triethylamine hydrochloride(9) White crystal (CH2Cl2),C6H16NCl,Rf= 0.58(CH2Cl2/5% MeOH).1H NMR(CD3OD,300 MHz)δ:3.28-3.19 (m,2H),1.53-1.15(m,3H).13C NMR(CD3OD,75 MHz)δ:47.723(S,CH2),9.33(S,CH3);EI-MS (m/z):101[M-HCl]+;HR-ESIMS(m/z:239.0[2M– Cl]+,297.0[2M+Na– H]+,calcd for C6H15N·HCl,137.50).According to checking the data of 1HNMR in AntiBase,compound 4 is C6H15N· HCl.
Result of brine shrimp bioassay
The results of toxicity test of isolated metabolites were presented in Table 1,DMSO was used to dissolve samples.Among ofnine compounds,only kalamycin showed marked toxicity toward brine shrimp larvae at the concentration of 40 μg/mL,with mortality rate of 90.5%.Maybe kalamycin was the main cytotoxic composition of this strain.

Table 1 Toxicity of nine compounds to the brine shrimp
Discussion
Nine compounds were isolated from the extract of fermented broth of Streptomycete sp.S007.Compound 9 was firstly obtained from marine streptomycete.Kalamycin showed marked toxic effect on brine shrimp larvae,it is the toxic active composition of this strain.
Acknowledgements We are grateful to Friederike Lissy at Department of Organic and Biomolecular Chemistry,Goetting Universtity for experimental technical assistance.
Reference
1 Zhao WY(趙文英),Zhu TJ(朱天嬌),Gu JY(古靜燕),et al.Studies on the chemical constituents and antitumor activity of secondary metabolites of marine-derived Streptomyces sp. 3275.Nat Prod Res Dev(天然產(chǎn)物研究與開發(fā)),2006,18: 405-407,417
2 Li FC,Maskey RP,Qin S,et al.Chinikomycin A and B:isolation,structure elucidation and biological activity of novel antibiotics from a marine Streptomyces sp.isolate M045.J Nat Prod,2005,68:349-353.
3 Maskey RP,Li FC,Qin S,et al.Chandrananimycins A~C: production of novelanti-cancer antibiotics from a marine actinomadura sp.isolate M048 by variation of medium composition and growth conditions.J Antibiot,2003,56:622-629.
4 Fotso S,Wu SJ,Qin S,et al.5,7-Dihydroxy-5,6,7,8-tetrahydro-1H-azocin-2-one from a marine-derived Streptomyces sp.Nat Prod Commun,2006,1:9-13.
5 Wu SJ,F(xiàn)otso S,Li FC,et al.N-carboxamido-staurosporine and Selina-4(14),7(11)-diene-8,9-diol,new metabolites from a marine Streptomyces sp.J Antibiot.2006,59:331-337.
6 Ding L,Pfoh R,Ruhl S,et al.T-Muurolol sesquiterpenes from the marine Streptomyces sp.M491 and revision of the configuration of previously reported amorphanes.J Nat Prod.2009,72:99-101.
7 Solis PN,Wright CW,Andersonetal MM,et al.A microwell cytotoxicity assay using artemia salina(brine shrimp).Planta Med,1993,59:250-252.
8 Meyer BN,F(xiàn)errigni NR,Putnam JE,et al.Brine shrimp:a convenient general bioassay for active plant constituents. Planta Med,1982,45(5):31-34.
9 Laatsch H.AntiBase,2002,A natural products database for rapid structure determination.ChemConcepts.Weinheim,2002,see Intemet:http://wwwuser.gwdg.de/-ucoc/laatsch/ antibase.htm
10 Elin RJ,Wolfe SM.Bacterial Endotoxin.In:Luskin AI,Lechevalier HA(eds.).CRC handbook of microbiology 1973,II: Boca Raton,CRC Press,pp 215-237.
11 Stueber D,Grant MD.13C and15N chemical shift tensors in adenosine,guanosine dihydrate,2'-deoxythymidine,and cytidine.J Am Chem Soc,2002,124:10539-10551
12 B?hlendorf B,Bedorf N,Jansen R,et al.Antibiotics from gliding bacteria,LXXIII indole and quinoline derivatives as metabolites of tryptophan in myxobacteria.Eur J Org Chem,1996,1:49-53.

