還原氧化石墨烯修飾Bi2WO6提高其在可見光下的光催化性能
應 紅 王志永 郭政鐸 施祖進,* 楊上峰
(1中國科學技術大學材料科學與工程系,合肥微尺度物質科學國家實驗室,中國科學院能量轉換材料重點實驗室,合肥230026;2北京大學化學與分子工程學院,稀土材料化學與應用國家重點實驗室,北京分子科學國家實驗室,北京100871)
還原氧化石墨烯修飾Bi2WO6提高其在可見光下的光催化性能
應 紅1,2王志永2郭政鐸2施祖進2,*楊上峰1,*
(1中國科學技術大學材料科學與工程系,合肥微尺度物質科學國家實驗室,中國科學院能量轉換材料重點實驗室,合肥230026;2北京大學化學與分子工程學院,稀土材料化學與應用國家重點實驗室,北京分子科學國家實驗室,北京100871)
通過兩步水熱法合成了一種新型的還原氧化石墨烯(RGO)修飾的Bi2WO6(Bi2WO6-RGO),結果表明其在可見光下的光催化性能得到了顯著的提高.研究了RGO在Bi2WO6-RGO中的含量對其光催化性能的影響,從而確定出RGO相對于Bi2WO6的最佳摻雜質量比值為1%.通過掃描電鏡(SEM)研究發現,RGO并沒有改變Bi2WO6光催化劑的結構和形貌.Bi2WO6-RGO在可見光下的光催化性能得以提高可以歸功于RGO.其可能的機理是石墨烯的存在有利于光生載流子(激子)的分離,從而導致產生更多的O2●-用于有機染料污染物(如羅丹明B(RhB))的降解.RhB分子在石墨烯上的有效吸附可能也是導致Bi2WO6-RGO光催化性能提高的另一原因.
Bi2WO6;石墨烯;水熱法;光催化劑;有機染料污染物
1 Introduction
In the past decade much attention have been paid to semiconductor as photocatalyst to degrade organic dye pollutant,1which was difficult to be biodegraded.2Nowadays,titanium dioxide(TiO2)has gained particular interest in this research field.3In spite of the success in converting light to chemical energy,TiO2could not be used widely and practically in the solar environment because its activation is limited to UV light.To utilize solar energy sufficiently especially those within visible region,intensive research has been concentrated on photocatalysts with high photocatalytic activity such as Bi2WO6,4-6BiVO4,7and CaIn2O4,8etc.In particular,Bi2WO6,with a high degree of mineralization and layered by WO6and[Bi2O2]2+layers,can lead to generation of active O2?-to decompose dye pollutant efficiently under visible light.4-6Bi2WO6with different structures,like three dimensional(3D)nanoparticles and two dimensional(2D)nanoplates,which could be synthesized through hydrothermal process by adjusting the pH value of the solution,shows diverse efficiency of photodegradation.9Lin et al.10reported that the efficiency of photodegradation was affected by the separation of photoexcited electron-hole and adsorption of organic molecules.In order to promote the separation of photogenerated electron-hole pairs,Zhu et al.11modified Bi2WO6with C60and found the synergetic effect of Bi2WO6photocatalyst with C60could enhance photoactivity efficiently. Li et al.12modified Bi2WO6with low cost carbon and improved the photocatalytic performance due to the enhanced photogenerated electron-hole separation and more RhB adsorption associated with carbon.
Graphene,as a new member of carbon family,is a zerobandgap semiconductor owing to its two-dimensional platelike structure.13In general,graphene has unique electronic properties,large specific surface area,and high transparency.13-15The reducing of graphene oxide(GO)is an easy and widely-used route to obtain graphene sheets,16named reduced graphene oxide(RGO).Graphene has been incorporated with photocatalyst as enhanced photodegradation composite under visible light.17-20TiO2-RGO composites were obtained through a simple hydrothermal process,and the excellent degradation of dye pollutants was ascribed to graphene?s adsorptivity,transparency,and conductivity.17Graphene-Au exhibited high visiblelight photocatalytic activity for dye degradation.18Recently, BiVO4was incorporated with RGO to enhance photoelectrochemical water splitting reaction.19More recently,Wang et al.20synthesized graphene-Bi2WO6composite via in situ hydrothermal reaction and found the enhanced photocatalytic activity.
In this study,we have synthesized RGO-modified Bi2WO6(Bi2WO6-RGO)as an improved photocatalyst via a two-step hydrothermal process.A series of Bi2WO6-RGO with different loading ratios of RGO was prepared and their photocatalytic activity towards the photodegradation of rhodamine-B(RhB) under visible light(≥420 nm)was investigated.
2 Experimental
2.1 Preparation of Bi2WO6-RGO
2.1.1 Synthesis of Bi2WO6
All chemical reagents were of analytical grade without further purification.Bi(NO3)3·5H2O,Na2WO4·2H2O,HNO3,and NaOH were used as the starting materials.Bi2WO6was synthesized by the hydrothermal method.Na2WO4·2H2O and Bi(NO3)3· 5H2O(the molar ratio is 1:2)were mixed together in deionized water(80 mL),followed by ultrasonication for 20 min.After that,the pH value of the solution was adjusted to 2 by adding 0.5 mol·L-1NaOH/HNO3.Then 80 mL of the final solution was transferred into a 100 mL Teflon-lined stainless steel autoclave.The autoclave was sealed and maintained at 180°C for 20 h.After the reaction,yellowish product was collected via repeated centrifugation and dispersion in water until pH value of the supernatant is 7 and then dried in an oven at 80°C.
2.1.2 Synthesis of GO
GO was prepared with the modified Hummers method.16Briefly,graphite was mixed with NaNO3and H2SO4in an icewater bath with stirring.KMnO4was added slowly into the previous mixture with stirring for 2 h again.After that,stirring vigorously for 5 days at room temperature was continued.With H2O2added into,the mixture was rinsed with a mixed aqueous solution of H2SO4/H2O2.Finally,the product was obtained after drying in an oven at 80°C.
2.1.3 Synthesis of Bi2WO6-RGO
Yellowish product Bi2WO6(1 g)was dispersed in 50 mL deionized water and then ultrasonicated for 1 h.Different content of GO(0.5%,1%,2%,mass ratio of GO to Bi2WO6)was dispersed rapidly in 30 mL ethanol with ultrasonic.Then the two solutions were mixed in a beaker under ultrasonic for 30 min and stirred for another 3 h to get a homogeneous suspension. Thereafter the 80 mL solution was transferred into the 100 mL Teflon-lined stainless steel autoclave and maintained in 160°C for 4 h.Then the light gray product was collected by filtration, rinsed some times by deionized water,and dried in an oven at 80°C.For comparison,blank Bi2WO6(without RGO)for photocatalytic experiment experienced this hydrothermal process again under the same condition without the addition of GO.
2.2 Characterization
High-resolution transmission electron microscopy (HRTEM)observations were carried out on Tecnai F20(America) with an accelerating voltage of 300 kV.The transmission electron microscopy(TEM)images were obtained by Hitachi H-9000(Japan)instrument.X-ray photoelectron spectroscopy (XPS)data were obtained using an ESCALab250 electron spectrometer from Thermo Scientific Corporation(America)with monochromatic 150 W Al Kαradiation and the base pressure was about 6.5×10-8Pa.The binding energies were referenced to the C 1s line at 284.8 eV from alkyl or adventious carbon. Scanning electron microscope(SEM)images were carried out on FEI NanoSEM 430 instrument(America).The information of crystalline structures of Bi2WO6and Bi2WO6-RGO were ob-tained by an X-ray diffractometer(XRD)(Rigaku Dmax2000, Japan).UV-visible absorption spectra were recorded on a UV-Vis spectrophotometer(Hitachi UV-3100,Japan)in the wavelength range of 400-700 nm.
2.3 Photocatalytic experiment
Photocatalytic activities of the Bi2WO6and Bi2WO6-RGO were evaluated by degradation of RhB under visible irradiation of a solar simulator(Newport 91160-1000,America)with a calibrated illumination power density of 100 mW·cm-2.The visible light was obtained through putting a cutoff filter(λ≥420 nm)between the lamp and the solution.In this experiment,0.1 g photocatalysts was added into 100 mL RhB solution(5×10-6mol·L-1).After stirring for 10 min in dark,the solution was exposed to visible light irradiation with stirring.Then 3 mL RhB solution was extracted each 30 min for UV-Vis absorption spectroscopic measurement.For comparison,the degradation of RhB irradiated without the addition of Bi2WO6-RGO under visible light(λ≥420 nm)and the degradation with the addition of Bi2WO6-RGO under dark condition were also measured as two reference experiments.
3 Results and discussion
Fig.1 shows the X-ray diffraction patterns of Bi2WO6with and without RGO,both of which exhibit characteristic peaks of Bi2WO6.Nearly identical peaks can be observed from the comparative figure of Bi2WO6and Bi2WO6-RGO(1%),indicating that the crystalline structure of Bi2WO6does not change during the reduction of GO in 160°C for 4 h.No any peak of RGO was observed in the XRD pattern of the Bi2WO6-RGO (1%)because of the small content of graphene used.
Fig.2 shows the C 1s XPS spectra of GO and Bi2WO6-RGO (1%).C 1s XPS spectrum generally contains three characteristic peaks corresponding to nonoxygenated C―C bond(284.5 eV),C―O(epoxy and hydroxyl)(286.6 eV),and carboxylate C=O from carboxylic acid(288.9 eV),respectively.19Fig.2 indicates that GO,which was prepared with the modified Hummers method,has a considerable degree of graphene oxidation. After 160°C hydrothermal process for 4 h in water and ethanol,obvious decrease of the intensities of C―O and C=O peaks of C 1s were observed for Bi2WO6-RGO(1%),indicating that efficient deoxygenation of GO occurred through the second hydrothermal process.
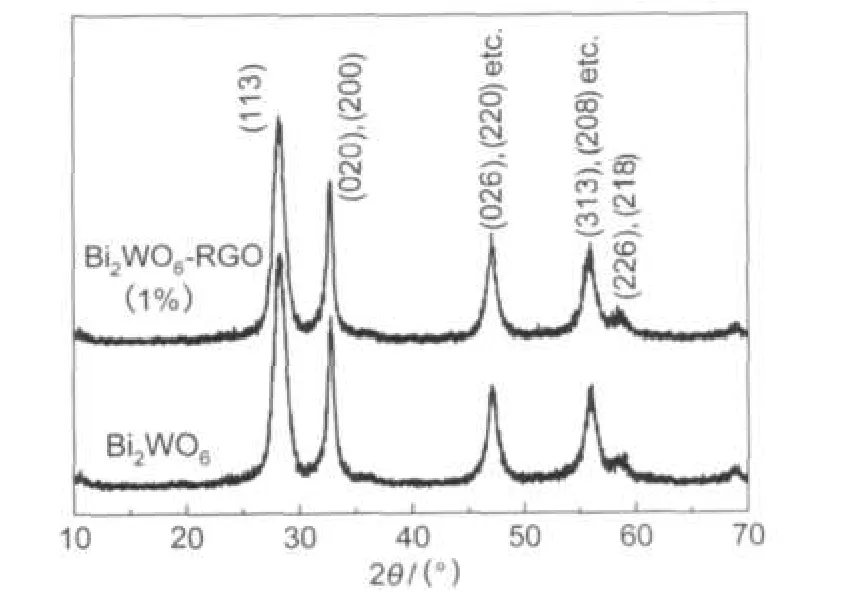
Fig.1 XRD patterns of Bi2WO6and Bi2WO6-RGO(1%)
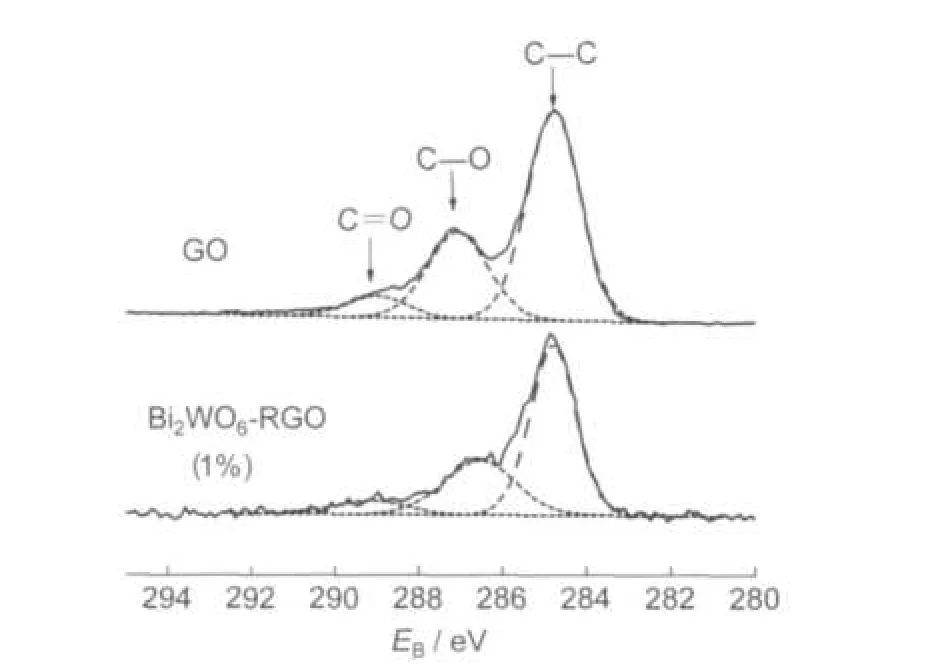
Fig.2 C 1s XPS spectra of GO and Bi2WO6-RGO(1%)
It has been reported that the photocatalytic activity of Bi2WO6for degradation of RhB under visible-light irradiation is dependent on the shape,size,and structure of the Bi2WO6.92D platelike structure could be changed to 3D flowerlike superstructure with the pH control or addition of surfactant.9From the low-magnification SEM image of Bi2WO6without RGO (Fig.3(a)),we can observe flowerlike spheres with coarse surface.The diameter of the flowerlike spheres is about 3 μm.For comparison,SEM image of Bi2WO6-RGO(1%)was given in Fig.3(b).The similar shape,diameter,and structure of macrospheres did not changed obviously from these images.However,while Bi2WO6precursor was transformed into crystal structure in hydrothermal process,existence of GO may disturb the crystalline structure and lead to different photocatalytic activity.6In our work,in order to exclude this additional influence, Bi2WO6with considerable photocatalytic activity would be obtained firstly and then modified with RGO for its excellent conductivity and transparency.According to the macroscopic shape of SEM images of these samples,the structure of Bi2WO6did not changed at all after RGO was attached to Bi2WO6.GO were adsorbed onto Bi2WO6plates or spheres firstly and then transformed to RGO with the reduction of ethanol and water via hydrothermal process.As the content of RGO was very low,the platelike structure of the graphene could not be observed in these SEM images.
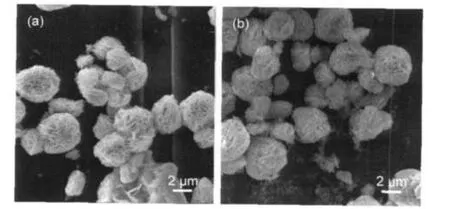
Fig.3 SEM images of Bi2WO6(a)and Bi2WO6-RGO(1%)(b)
More structure details of the Bi2WO6-RGO were obtained from TEM and HRTEM observations(Fig.4).The“graphene cloth”(RGO looks like a cloth)tangling with the Bi2WO6particle was observed in the low magnification TEM image (Fig.4a).The high-magnification HRTEM image(inset of Fig.4b)exhibits a group of clear parallel crystal planes,which ascribe to(200)faces with interspacing of 0.272 nm.And the poorly-crystallized carbon layer adhering to the surface of Bi2WO6particle is very clear in the HRTEM image(Fig.4b). From these TEM and HRTEM images we can infer that Bi2WO6was modified by RGO and the composite photocatalyst was obtained.The presence of these“graphene cloth”is important to improve the photoactivity because the excited electron of Bi2WO6in the visible irradiation could be transferred to graphene rapidly.
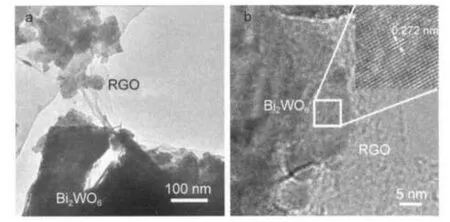
Fig.4 TEM(a)and HRTEM(b)images of Bi2WO6-RGO(1%)Inset is the magnification of labeled area.
The photocatalytic activity of Bi2WO6-RGO with different contents of RGO was evaluated by the degradation of RhB in aqueous solution under visible light.RhB showed a major absorption band at 553 nm.Before irradiation,the photocatalyst and RhB solution mixture were stirred for 10 min,then the adsorption equilibrium of RhB on photocatalyst was reached.As the irradiation time increases,RhB is de-ethylated step by step.21During this process,the absorption intensity of 533 nm peak decreases gradually and the characteristic peaks shifts to shorter wavelength with the color changing from pink to light green.Photocatalytic degradation of RhB by Bi2WO6with different mass ratios of RGO to Bi2WO6(0%,0.5%,1%,2%)under visible irradiation was presented in Fig.5a.With the increase of the content of RGO,the photoactivity towards degradation of RhB is enhanced gradually at first for 0.5%-1%loading ratio but decreased with the higher loading ratio of 2%. With irradiation,Bi2WO6-RGO(1%)photocatalyst shows the highest velocity of photodegradation among different photocatalysts under visible light.This result is similar to C60-modified Bi2WO6,for which the optimum synergetic effect of C60and Bi2WO6is reported at a mass ratio of 1.25%(C60to Bi2WO6).11Fig.5b shows the UV-Vis absorption spectra of RhB during the photodegradation with Bi2WO6-RGO(1%)as the photocatalyst under visible light for different irradiation time.Graphene with appropriate content could accept the photogenerated electrons of Bi2WO6,and reduce O2to O2?-rapidly which promotes the degradation of dyes.11Meanwhile,graphene enhances the adsorption of RhB,which also improves the degradation of dye pollutants.However,when the mass ratio of RGO to Bi2WO6reaches to 2%,the degradation of RhB slows down dramatically.This phenomena is interpreted by two reasons. First and mainly,thick and dense graphene layer could inhibit the inherent optical absorption of Bi2WO6and finally reduce the photoactivity.12Second,the excess GO could not be reduced entirely during the hydrothermal process,and thus the excited electron of Bi2WO6,which can reduce O2to O2?-,is used to reduce C=O and C―O of the remnants GO.22
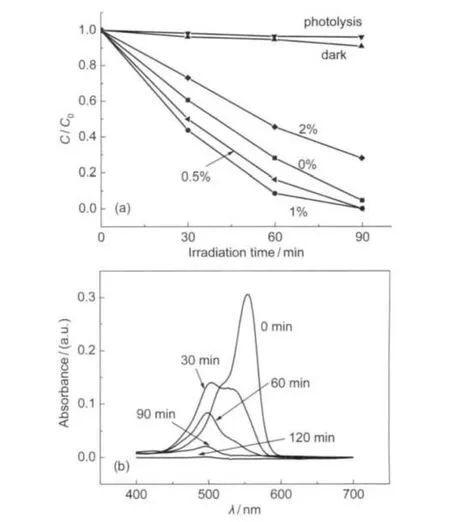
Fig.5 (a)Photocatalytic degradation of Bi2WO6-RGO with different mass ratios of RGO under visible light irradiationC0and C are the initial concentration of RhB under adsorption equilibrium and the concentration of RhB at different irradiation time,respectively.For comparison,“dark”is photodegradation curve of Bi2WO6-RGO(1%)in dark,“photolysis”is photodegradation curve without the addition of any photocatalyst under visible light.(b)UV-Vis absorption spectra of RhB during the photodegradation with Bi2WO6-RGO(1%)as the photocatalyst under visible light for different irradiation time
In order to identify the role of RGO in improving the photocatalytic activity of Bi2WO6,the experiments should be carried out between pure Bi2WO6and Bi2WO6-RGO composite with identical crystal structure and macroscopic shape of Bi2WO6.It should be noted that,recently,Wang et al.20synthesized graphene-Bi2WO6composite through a similar hydrothermal method and found much more enhanced photocatalytic activity towards photodegradation of RhB in terms of the shorter degradation time.The nano size of Bi2WO6is the main reason for shortening degradation time to several minutes.Nevertheless, the major difference between the work in Ref.20 and our present work is that the Bi2WO6crystal in Ref.20 is formed in the presence of GO(in situ).It is known that the structure and mor-phology of Bi2WO6would be changed with an additive like surfactant.9GO,as a water-soluble additive,might also change the structure and morphology of Bi2WO6and thereby lead to a different photoactivity.Besides,Bi3+as metal ion would be adsorbed by carboxylic acid functional groups of the GO easily.23Consequently,GO would be aggregated in the existence of Bi3+between graphene layers.The aggregated graphene might decrease light absorption of Bi2WO6.To exclude the possibility that different photoactivity is originated from different structure of Bi2WO6instead of graphene modification,Bi2WO6was preferably prepared first and RGO was introduced in the secondstep hydrothermal process in our work.Hence in this way the improvement of photoactivity of Bi2WO6-RGO can be undoubtedly ascribed to RGO.
4 Conclusions
Bi2WO6-RGO was synthesized by a two-step hydrothermal process:the first step is to obtain Bi2WO6with controllable microstructure,and in the second step GO is reduced and attached to Bi2WO6.The application of Bi2WO6-RGO in photocatalytic degradation of RhB is studied.The modification of RGO does not affect the structure and morphology of Bi2WO6as confirmed from XRD and SEM studies,which is important for elucidating the role of RGO.Graphene accepts photogenerated electrons of Bi2WO6efficiently under visible light and promotes the dissociation of photogenerated excitons of Bi2WO6.More electrons on the graphene lead to faster reduction of O2to O2?-which promotes the degradation of RhB.Moreover,the existence of RGO on the surfaces of Bi2WO6particles can enhance the adsorption of RhB.Effect of content of RGO on the photoactivity was discussed and the optimum loading ratio was determined to be 1%(mass ratio of RGO to Bi2WO6). Nevertheless,the photoactivity decreases when the mass ratio of RGO to Bi2WO6is 2%,which may be caused mainly by the decrease of optical absorption of photocatalyst under visible light.The RGO-modified Bi2WO6with improved photoactivity prepared in this work has a high potential as the photocatalyst in environment remediation.
(1)Hoffmann,M.R.;Martin,S.T.;Choi,W.Y.;Bahnemannt,D. W.Chem.Rev.1995,95,69.
(2) Raffainer,I.I.;von Rudolf,R.P.Ind.Eng.Chem.Res.2001,40, 1083.
(3) Agrios,A.G.;Pichat,P.J.Appl.Electrochem.2005,35,655.
(4) Kudo,A.;Hijii,S.Chem.Lett.1999,28,1103.
(5)Tang,J.W.;Zou,Z.G.;Ye,J.H.Catal.Lett.2004,92,53.
(6) Zhang,C.;Zhu,Y.F.Chem.Mater.2005,17,3537.
(7)Kudo,A.;Omori,K.;Kato,H.J.Am.Chem.Soc.1999,121, 11459.
(8)Tang,J.W.;Zou,Z.G.;Ye,J.H.Chem.Mater.2004,16,1644.
(9) Zhang,L.S.;Wang,W.Z.;Zhou,L.;Xu,H.L.Small 2007,3, 1618.
(10)Lin,X.P.;Huang,T.;Huang,F.Q.;Wang,W.D.;Shi,J.L. J.Mater.Chem.2007,17,2145.
(11) Zhu,S.B.;Xu,T.G.;Fu,H.B.;Zhao,J.C.;Zhu,Y.F.Environ. Sci.Technol.2007,41,6234.
(12) Li,Y.Y.;Liu,J.P.;Huang,X.T.;Yu,J.G.Dalton Trans.2010, 39,3420.
(13) Kamat,P.V.J.Phys.Chem.Lett.2010,1,520.
(14) McAllister,M.J.;Li,J.L.;Adamson,D.H.;Schniepp,H.C.; Abdala,A.A.;Liu,J.;Herrera-Alonso,M.;Milius,D.L.;Car, R.;Prud?homme,R.K.;Aksay,I.A.Chem.Mater.2007,19, 4396.
(15) Nair,R.R.;Blake,P.;Grigorenko,A.N.;Novoselov,K.S.; Booth,T.J.;Stauber,T.;Peres,N.M.R.;Geim,A.K.Science 2008,320,1308.
(16) Hontoria-Lucas,C.;Lopez-Peinado,A.J.;Lopez-Gonzaiez,J. D.;Rojas-Cerantes,M.L.;Martin-Aranda,R.M.Carbon 1995, 33,1585.
(17)Zhang,H.;Lv,X.J.;Li,Y.M.;Wang,Y.;Li,J.H.ACS Nano 2009,4,380.
(18) Xiong,Z.G.;Zhang,L.L.;Ma,J.Z.;Zhao,X.S.Chem. Commun.2010,46,6099.
(19) Ng,Y.H.;Iwase,A.;Kudo,A.;Amal,R.J.Phys.Chem.Lett. 2010,1,2607.
(20)Gao,E.P.;Wang,W.Z.;Shang,M.;Xu,J.H.Phys.Chem. Chem.Phys.2011,13,2887.
(21) Li,Y.Y.;Liu,J.P.;Huang,X.T.Nanoscale Res.Lett.2008,3, 365.
(22)Williams,G.;Seger,B.;Kamat,P.V.ACS Nano 2008,2,1487.
(23)Zhang,L.;Wang,W.Z.;Shang,M.;Sun,S.M.;Xu,J.H. J.Hazard.Mater.2009,172,1193.
January 18,2011;Revised:March 23,2011;Published on Web:May 9,2011.
Reduced Graphene Oxide-Modified Bi2WO6as an Improved Photocatalyst under Visible Light
YING Hong1,2WANG Zhi-Yong2GUO Zheng-Duo2SHI Zu-Jin2,*YANG Shang-Feng1,*
(1Key Laboratory of Materials for Energy Conversion,Chinese Academy of Sciences,Hefei National Laboratory for Physical Sciences at Microscale,Department of Materials Science and Engineering,University of Science and Technology of China,Hefei 230026,P.R.China;2Beijing National Laboratory for Molecular Sciences,State Key Laboratory of Rare Earth Materials Chemistry and Applications,College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China)
A new and improved photocatalyst,reduced graphene oxide(RGO)-modified Bi2WO6(Bi2WO6-RGO),was synthesized by a two-step hydrothermal process.The effect of RGO content on photoactivity was investigated and the optimum mass ratio of RGO to Bi2WO6was determined to be 1%. Based on scanning electron microscopic study,RGO does not change the structure and morphology of the Bi2WO6photocatalyst.Therefore,the improvement in the photoactivity of the Bi2WO6-RGO composite is undoubtedly ascribed to RGO.The presence of graphene can facilitate the dissociation of photogenerated excitons,which leads to more O2●-to degrade dye pollutants like rhodamine-B(RhB).Moreover,the efficient adsorption of RhB molecules on graphene is another reason for the improved photoactivity.
Bi2WO6;Graphene;Hydrothermal process;Photocatalyst;Dye pollutant
?Corresponding authors.SHI Zu-Jin,Email:zjshi@pku.edu.cn;Tel:+86-10-62751495.YANG Shang-Feng,Email:sfyang@ustc.edu.cn; Tel:+86-551-3601750.
The project was supported by the National Natural Science Foundation of China(20771010,20801052),National Key Basic Research Program of China(973)(2011CB932601),National High Technology Research and Development Program of China(863)(2007AA03Z311),and“100 Talents Program of ChineseAcademy of Sciences”(A1010).
國家自然科學基金(20771010,20801052),國家重點基礎研究發展規劃項目(973)(2011CB932601)國家高技術研究發展計劃項目(863) (2007AA03Z311)和中國科學院百人計劃(A1010)資助
O643;O645

