Synthesis,Crystal Structure and Nonlinear Optical Properties of a Novel Imidazole Derivative
CHENG Le-HuaCHU Xian-PingWANG MinWANG Hai-Yan CHEN Hong
(1 Department of Chemistry and Material Science,ChaoHu college,ChaoHu Anhui 238000)
(2 Institution of Coordination Chemistry,ChaoHu college,ChaoHu Anhui 238000)
Synthesis,Crystal Structure and Nonlinear Optical Properties of a Novel Imidazole Derivative
CHENG Le-Hua1,2CHU Xian-Ping1,2WANG Min1,2WANG Hai-Yan1,2CHEN Hong1,2
(1 Department of Chemistry and Material Science,ChaoHu college,ChaoHu Anhui 238000)
(2 Institution of Coordination Chemistry,ChaoHu college,ChaoHu Anhui 238000)
A new compound 1-[trans-4-((N-ethylcarbazolyl)vinyl)phenyl]imidazole(C25H22N3,Mr=364.46)has been synthesized,and its crystal structure was determined by single-crystal X-ray diffraction method.The crystal is of monoclinic,space group P21/c with a=9.298(2) ,b=8.648(2) ,c=25.394(7) ,β=110.46(3)°,V=1912.9(8)3,Z=4,Dc=1.266g/cm3,μ=0.075mm-1,F(000)=772,The structure was refined to R=0.0685 and wR=0.1612 for 3570 unique reflections with Rint=0.0645.The structural determination shows that the molecular structure is a perfectly planar π-conjugated system, and there is a high electronic delocalization in the molecule.The compound shows photoluminescence with a maximum emission at 439nm and a shoulder emission at 458nm upon the maximum-excitation wavelength at 264nm.
imidazole derivative;crystal structure;π-conjugated system;photoluminescence
1INTRODUCTION
In recent years,there has been growing interest in the design and synthesis of organic linear or nonlinear optical materials due to their potential applications in many fields, such as three-dimensiona imaging,optical data storage,three-dimensional lithographic microfabrication and photodynamic therapy,etc[1-6].The molecules with large polar πconjugated system and donor-acceptor substituents often have excellent linear or nonlinear optical properties[7-10].Our group have successfully prepared and studied a series of compounds with π-conjugated system. In our present work, a novel imidazole derivative,1-[trans-4-((N-ethyl carbazolyl) vinyl)phenyl]imidazole,with D-π-A structure has been prepared,using imidazole and carbazole as A and D units,respectively.We herein firstly report the synthesis,crystal structure and nonlinear
2EXPERIMENTAL
2.1 Materials and measurements
All regents were obtained from commercial sources and used without further purification.1H NMR spectrum(DMSO)was performed on EM500 with TMS as an internal standard.IR spectra were recorded from KBr discs in the range of 4000~400cm-1on a Nicolet FT-IR-intrument.Elemental analysis was performed on Perkin-Elmer 240 analyzer.UV-vis spectra were measured in DMF solutions(10-5mol·L-1)with a UV-265 spectrophotometer.Luminescence spectra were recorded on a Perkin-Elmer LS55B fluorescence spectrophotometer at room temperature.
2.2 Synthesis
2.2.1 Synthesis of[(N-ethy carbazoly)methylene]thriphenylposphoniumiodide
N-ethyl-3-formylcarbazole and [(N-ethylcarbazole)methylene]thriphenyl-phosphonium iodide were synthesized according to the methods reported[1,11,12].Anal.Calc.(%)for C33H29NPI:C,66.34;H,4.89;N,2.34.Found(%):C,66.49;H,4.55;N,2.21.ESI-MS:m/z=470.2.
2.2.2 Synthesis of 4-imidazoly-benzaldehyde
60 mL of DMF,6.8g (0.1mol)of imidazole,13.8g(0.1mol)of anhydrous potassium carbonate,and three drops of aliquate-336 were introduced into a round-bottom flask.The mixture was stirred for 10 min at 90℃.Then 12.4g(0.1mol)of 4-fluorobenzaldehyde was added dropwise to above solution.The mixture turned yellow and was kept stirring for 24h at 85℃.After cooling to room temperature,it was poured into 200 mL of ice-water mixture, and then the pale-yellow lamellar crystals formed immediately. The product was filtered off and dried in vacuo over P2O5.The total yield of the product was 15.5g(90%). Anal. Calc.(%) for C10H8N2O:C,63.58;H,5.96;N,9.27.Found(%):C,63.50;H,6.10;N,9.21.MS:m/z=172.
2.2.3 Synthesis of 1-[trans-4-((N-ethy1 carbazoly1)viny1)pheny1]imidazole
5.97 g (10mmol) of[(N-ethy1 carbazoly1)methylene]thripheny1 phosphonium iodide, 1.72g(10mmol)of 4-imidazoly1-benzaldehyde and 2.00g(50mmol) of NaOH were placed in a mortar.The mixture was grinded forcibly for 20min,then poured into 500mL of distilled water.The product was extracted twice with CH2Cl2, and the organic layer was dried overnight over anhydrous MgSO4. The solvent was removed with a rotary evaporator to give the crud product.The yellow product was purified by column chromatography on silicagel using ethyl acetate-petroleum ether (4:1) as eluent.Yield: 2.9g(80%).Anal.Calc.(%)for C25H22N3:C,82.64;H,5.79;N,11.57.Found(%):C,82.55;H,5.92;N,11.69.ESI-MS:m/z=364.3
(HM+).IR(KBr,cm-1):1621(m),1595(s),1517(s),1482(s),1301(m).1HNMR:
δH1.3(s,3H,-CH3),3.3(m,2H,-CH2-),6.6(m,2H,HC=CH).UV-vis,λmax(nm)(DMF):345(logε=4.32).
Single crystal of title compound was isolated by slow evaporation of a benzene solution at room temperature.
2.3 Crystal date and structure determination
A yellow crystal with dimension of 0.35mm×0.20mm×0.18mm was selected and mounted on a glass fiber in air.The diffraction data were collected at 293K on a Bruker SMART CCD diffractometer with graphite monochromated MoKα radiation (λ=0.71073).A total of 8011 reflections were collected in the range of 1.71 < θ < 25.50°by using a ω scan mode,of which 3570 were unique with Rint=0.0645,and 3570 observed reflections with I>2σ (I)were used in the succeeding structure calculations.The intensity data were corrected for Lorentz-polarization factors,and empirical absorption correction was applied.The structure was solved by direct methods and difference Fourier syntheses.The non-hydrogen atoms were refined anisotropically, and hydrogen atoms were introduced geometrically.The final refinement of full-matrix least-squares for 3570 unique reflections with I>2σ(I)was converged to R=0.0685,wR=0.1612.The highest and lowest residual peaks in the final difference Fourier map are 0.420and –0.178e/A3,respectively.All calculations were performed with SHELXTL-97 package[13].
3 RESULTS AND DISCUSSION
3.1 Synthesis and characterization
Scheme 1 describes the synthetic route of the title compound,1-[trans-4-((N-ethy1 carbazolyl)vinyl)phenyl]imidazole,which was synthesized by modified solid-state Witting reaction with a high yield. Wittig reaction is a widely used method to synthesize trans-alkene.Conventional Wittig reaction are carried out in solution and in N2atmosphere using strong base,such as MeONa,t-BuOK and even NaH,NaNH2,etc.In our experiment,we ground the reaction mixture in a mortar,using the commom base-NaOH.The reaction could be accomplished within half an hour and the product could be hadled easily and the yield was above 50%.So solid-state Wittig reaction is a wonderful,inexpensive and convenient method to synthesize trans-alkene[12].UV-vis spectrum of the compound displays absorption peak at 345nm,which are assigned as K band absorption arising from π→π*transitions within the extended π-conjugated system.The large molar absorption coefficient(logε=4.32)is also the indication of high electronic delocalization system.
3.2 Structural description
The atomic coordinates and equivalent isotropic displacement parameters for the title compound are given in Talbe1,and the selected bond lengths and bond angles in Tables2 and 3,respectively.Fig.1 shows the molecular structure of the title compound,and the packing diagram is shown in Fig.2. In the crystal, the molecules are stacked through strong π-π interaction with the distance of 3.090between two neighboring molecules.In the molecule,the dihedral angle between the imidazole and benzene ring is 19.9°.The benzene and carbazole ring form a dihedral angle of 25.0°.The sum of the three angles(C(1)-C(6)-C(5),123.1(5°);C(5)-C(6)-N(1),128.8(5°);C(1)-C(6)-N(1),108.1(4)°)is 360°,and the sum of the three angles(C(10)-C(9)-C(14),120.3(5)°;C(10)-C(9)-N(1),131.1(5)°;C(14)-C(9)-N(1),108.6(4)°)is also 360°,therefore the carbazole ring is perfectly planar.It can be seen from Table2 that almost bond lengths of C-C,except C(24)-C(25)(1.326(6))are located between thenormal C-C single bond(1.54)and C-C doublebond(1.34 ).Except N(1)-C(7)(1.476(5)),all C-N bond lengths are located between the normalC-N single bond (1.47 )and C-N double bond(1.33 ). The average bond lengths show the molecule is a π-conjugated system,which will be benefit to the intramolecular charge transfer and the enhancement of TPA cross sections[14].
3.3 Photoluminescence
The title compound exhibits strong photoluminescence in solid state. The photoluminescence properties of title compound show that the compound shows photoluminescence with a maximum emission at 439nm and a shoulder emission at 458nm upon the maximum-excitation wavelength at 264nm in Fig.3.The maximum emixxion is assigned to the π→π*transitions of the π-conjugated system, and the shoulder emission may arise from n→π transitions.

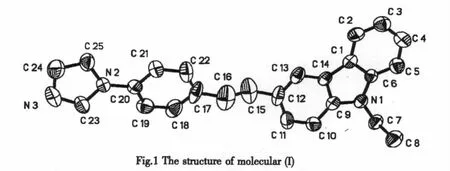
Table1 Atomic coordinates(×104)and equivalent isotropic displacement parameters(2×103)
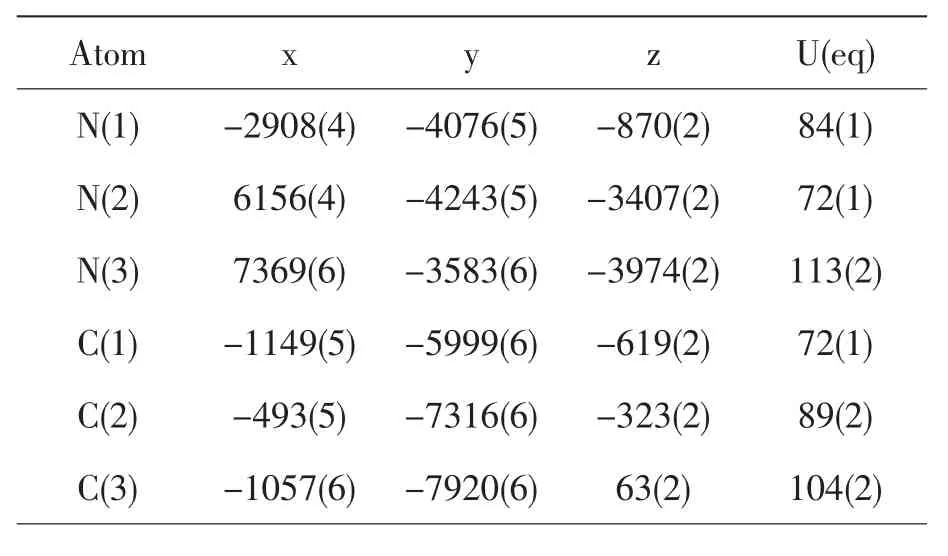
Table1 Atomic coordinates(×104)and equivalent isotropic displacement parameters(2×103)
Atom x y z U(eq)N(1) -2908(4) -4076(5) -870(2) 84(1)N(2) 6156(4) -4243(5) -3407(2) 72(1)N(3) 7369(6) -3583(6) -3974(2) 113(2)C(1) -1149(5) -5999(6) -619(2) 72(1)C(2) -493(5) -7316(6) -323(2) 89(2)C(3) -1057(6) -7920(6) 63(2) 104(2)

C(4) -2289(7) -7254(7) 157(2) 107(2)C(5) -2981(5) -5949(7) -128(2) 95(2)C(6) -2404(5) -5357(6) -517(2) 72(1)C(4) -2289(7) -7254(7) 157(2) 107(2)C(5) -2981(5) -5949(7) -128(2) 95(2)C(6) -2404(5) -5357(6) -517(2) 72(1)C(7) -4224(5) -3077(6) -899(2) 98(2)C(8) -3745(5) -1776(6) -496(2) 118(2)C(9) -1968(5) -3912(6) -1194(2) 75(1)C(10) -1997(6) -2838(6) -1607(2) 96(2)C(11) -943(8) -2988(7) -1867(2) 111(2)C(12) 148(7) -4175(9) -1743(3) 109(2)C(13) 165(5) -5193(6) -1333(2) 91(2)C(14) -884(5) -5106(5) -1055(2) 72(1)C(15) 1331(9) -4347(8) -2002(3) 168(3)C(16) 1673(10) -4001(9) -2362(3) 174(4)C(17) 2877(8) -4179(8) -2607(4) 125(3)C(18) 2586(7) -3552(7) -3133(3) 120(2)C(19) 3632(6) -3597(6) -3389(2) 98(2)C(20) 5053(5) -4258(5) -3142(2) 73(1)C(21) 5360(5) -4930(5) -2617(2) 86(2)C(22) 4287(7) -4898(6) -2362(2) 110(2)C(23) 6188(6) -3310(6) -3826(2) 98(2)C(24) 8124(6) -4756(8) -3654(3) 105(2)C(25) 7387(6) -5168(6) -3313(2) 101(2)
Table2 Selected Bond lengths]
Table2 Selected Bond lengths]
Bond Dist Bond Dist N(1)-C(6) 1.400(5) N(1)-C(9) 1.400(5)N(1)-C(7) 1.479(5) N(2)-C(23) 1.345(5)N(2)-C(25) 1.348(5) N(2)-C(20) 1.411(5)N(3)-C(2) 1.300(5) N(3)-C(24) 1.336(6)C(1)-C(2) 1.383(6) C(1)-C(6) 1.395(5)C(1)-C(14) 1.439(6) C(2)-C(3) 1.369(6)C(3)-C(4) 1.375(6) C(4)-C(5) 1.373(6)C(5)-C(6) 1.377(6) C(7)-C(8) 1.481(6)C(9)-C(10) 1.394(6) C(9-C(14) 1.399(4)C(10)-C(11) 1.366(6) C(11)-C(12) 1.400(7)C(12)-C(13) 1.360(7) C(12)-C(15) 1.472(7)C(13)-C(14) 1.392(6) C(15)-C(16) 1.351(6)C(16)-C(17) 1.456(9) C(17)-C(18) 1.379(8)C(17)-C(22) 1.387(8) C(18)-C(19) 1.345(6)C(19)-C(20) 1.374(6) C(20)-C(21) 1.389(5)C(21)-C(22) 1.366(6) C(24)-C(25) 1.326(6)
Table3 Selected Bond Angles()

Table3 Selected Bond Angles()
Angle (°) Angle (°)C(6)-N(1)-C(9) 108.6(4) C(6)-N(1)-C(7) 125.4(4)C(9-)-N(1)-C(7) 126.0(4)C(23)-N(2)-C(25)104.4(4)C(23)-N(2)-C(20)127.2(5)C(25)-N(2)-C(20)128.7(5)C(24)-C(25)-N(2)109.0(5) C(2)-C(1)-C(6) 117.7(5)C(2)-C(1)-C(14) 134.2(5) C(6)-C(1)-C(14) 108.0(5)C(19)-C(20)-N(2)121.5(5)C(22)-C(17)-C(16)126.9(8)C(18)-C(17)-C(16)116.7(8)C(18)-C(17)-C(22)116.4(5)C(5)-C(6)-C(1) 123.1(5) C(5)-C(6)-N(1) 128.8(5)C(1)-C(6)-N(1) 108.1(4)C(19)-C(20)-C(21)117.1(4)C(10)-C(9)-C(14)120.3(5) C(10)-C(9)-N(1) 131.1(5)C(14)-C(9)-N(1) 108.6(4)C(11)-C(10)-C(9)118.2(5)C(21)-C(20)-N(2)121.5(4)C(13)-C(12)-C(11)117.4(5)C(13)-C(12)-C(15)117.8(7)C(11)-C(12)-C(15) 124.7 C(16)-C(15)-C(12)144.6(11)C(13)-C(14)-C(9)118.9(5)C(9)-C(14)-C(1) 106.8(4)C(13)-C(14)-C(1)134.4(5)
REFERENCES:
[1]Zheng,Q.D,He,G.S,Lin,T.C,Prasad,P.N.J.Mater.Chem.2003,(13):2499-2504.
[2]Lu,Y,Hasegawa,F,Goto,T,Ohkuma,S,Fukuhara,S,Kawazu,Y,Totani,K,Yamashita,T,Watanabe,T.J.Mater.Chem.2004,(14):75-80.
[3]He,G.S,Cui,Y.P,Bhawalkar,J.D,Prasad,P.N,Bhawalkar,D.D.Optics Communications 1997,(133):175-179.
[4]Wang,X.M,Wang,D,Zhou,G.Y,Yu,W.T,Zhou,Y.F,Fang,Q,Jiang,M.H.J.Mater.Chem.2001,(11):1600-1605.
[5]Belfied,K.D,Ren,X,Miesak,J.J.Am.Chem.Soc.2000,(122):1217-1218.
[6]Rumi,M,Ehriliich,J.E,Perry,J.W.J.Am.Chem.Soc.2000,(122):9501-9510.
[7]Bunning,T.J,Kirkpatrick,s.m,Tomlin,D.W.Chem.Mater.2000,(12):2842-2844.
[8]Ren,Y,Fang,Q,Yu,W.T,Lei,H,Tian,Y.P,Jiang,M.H,Yang,C.Y,Mak,T.C.W.J.Mater.Chem.2000,(10):2025-2030.
[9]Abbotto,A,Beverina,L,Bradamante,S,Facchetti,A,Pagani,G.A,Bozio,R,Ferrante,C,Pedron,D,Signorini,R.Synthetic Metals 2003,(136):795-797.
[10]Liu,Z.Q,Fang,Q,Wang,D,Cao,D.X,Xue,G,Yu,W.T,Lei,H.Chem.Eur.J.2003,(9):5074-5084.
[11]Zhao C.F,Park C.K,Prasad P.N,Chem.Mater.1995,(7):1237.
[12]Yan Y.X,Journal of Solid State Chemistry,2003,(172):364.
[13]She1drick,G.M.SHELXTL V51 Software Reference Manual,Bruker AXS,Inc,Madison,Wisconsin,USA.1997.
[14]Zhang,X,Yu,X.Q,Yu,W.T,Zhao,H.P,Jiang,M.H.Chin.J.Struct.Chem.2005,(6):660-672.
0621
A
1672-2868(2011)06-0088-05
2011-10-05
This work was supported by a grants for the Education Committee of Anhui Province(KJ2010B126)
Corresponding author.Cheng le-hua.born in 1969,vice-professor,majoring in Coordination Chemistry
責(zé)任編輯:宏 彬

