一種三核銅(Ⅱ)配合物{[Cu(HL)(EtOH)]2Cu}的合成及結構研究
丁玉潔 王 揚
(1安徽工程大學生物與化學工程學院,蕪湖 241000)
(2國家知識產權局專利局材料部,北京 100088)
一種三核銅(Ⅱ)配合物{[Cu(HL)(EtOH)]2Cu}的合成及結構研究
丁玉潔*,1王 揚2
(1安徽工程大學生物與化學工程學院,蕪湖 241000)
(2國家知識產權局專利局材料部,北京 100088)
通過雙肟配體H4L(H4L=6,6′-二羥基-2,2′-[1,2-亞乙基二氧雙(氮次甲基)]二酚)與水合乙酸銅反應,合成了一個新的銅配合物{[Cu(HL)(EtOH)]2Cu},并進行了元素分析、紅外光譜和X-射線單晶衍射測定。結果表明,該配合物為三核結構,由3個CuⅡ離子、2個配位的(HL)3-單元、2個配位的乙醇分子組成。且配合物通過分子間C-H…O氫鍵形成了一個二維超分子結構。
雙肟配體;CuⅡ配合物;合成;晶體結構
H2salen and its metal complexes have been discovered since 19th century,unique functionalities such as host-guest chemistry,asymmetric catalysis, electronic conductivity,magnetism have been focused on these metal complexes[1-3]. Because the high electronegative oxygen atoms affect strongly azomethine nitrogen and N2O2coordination plane on the basis of N2O2H2salen-type bisoxime ligands,which can lead to different and novel structures of the resulted complexes[4]. The studies of H2salen-type bisoxime ligands and their complexes have made enormous progress in recent years[5-9].In addition,these complexes display novel magnetic,structural and redox properties which will continue to make them more interesting[10-12]. Herein,a new trinuclear CuⅡcomplex{[Cu(HL)(EtOH)]2Cu}with H4L(H4L=6,6′-dihydroxy-2,2′-[(1,2-ethylenedioxybis-(nitrilomethylidyne)]diphenol), has been synthesized and characterized structurally.
1 Experimental
1.1 Reagents and physical measurements
3-Hydroxysalicylaldehyde (≥98%)from Alfa Aesar was used without further purification.The other reagents and solvents were analytical grade reagents from Tianjin Chemical Reagent Factory,and were used without further purification.
Elemental analysis for CuⅡwas performed by an IRIS ER/S·WP-1 ICP atomic emission spectrometer.C, H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer.FTIR spectra were recorded on a VERTEX70 FTIR spectrophotometer with KBr pellet(400~4 000 cm-1) and CsI(100~500 cm-1)pellets.X-ray single crystal structure was determined on a Bruker Smart 1000 APE X CCD area detector.Melting points were measured by the use of a microscopic melting point apparatus made in Beijing Taike Instrument Limited Company,and the thermometer was uncorrected.Molar conductance value measurement was carried out on a model DDS-11D type conductivity bridge using 1.0 mmol·dm-3solution in DMF at 25℃.TG-DTA analysis was carried out on a Mettler Toledo Starethermoanalyzer at a heating rate of 5℃·min-1(N2atmosphere).
1.2 Synthesis and characterization of the ligandH4L
The major reaction steps involved in the synthesis of 6,6′-dihydroxy-2,2′-[(1,2-ethylenedioxybis(nitrilomethylidyne)]diphenol(H4L)are given in Fig.1.
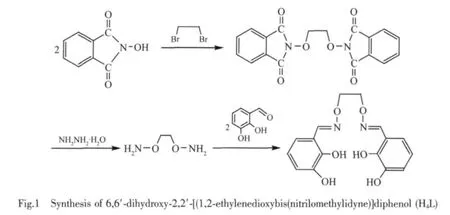
1,2-Bis(phthalimidoxy)ethane and 1,2-bis(aminooxy)ethane were synthesized according to the literature methods[5-6,8].
H4L was synthesized according to an analogous method reported earlier[13-14].To an ethanol solution(5 mL)of 3-hydroxysalicylaldehyde(138.3 mg,1.00 mmol) was added dropwise an ethanol solution (5 mL)of 1,2-bis(aminooxy)ethane(46.1 mg,0.50 mmol)(Fig.1).The mixture solution was stirred at 55℃ for 5 h.After cooling to room temperature,the precipitate was filtered off,and washed successively with ethanol and ethanolhexane(1∶4,V/V).The product was dried in vacuo and purified by recrystallization from ethanol to yield 223.1 mg(Yield,90.9%)of colorless microcrystal;m.p.111~112℃.1H NMR (400 MHz,CDCl3,δ,ppm):4.51(s, 4H),5.55(s,2H),6.73(dd,J=7.9,1.6 Hz,2H),6.84(t, J=7.9 Hz,2H),6.98 (dd,J=7.9,1.6 Hz,2H),8.23(s, 2H),9.89(s,2H).Anal.Calcd.for C16H16N2O6(H4L)(%): C,57.83;H,4.85;N,8.43;Found(%):C,57.85;H, 4.82;N,8.44.
1.3 Synthesis and characterization of the complex {[Cu(HL)(EtOH)]2Cu}
The single crystal of{[Cu(HL)(EtOH)]2Cu}was obtained by means of gaseous diffusion method.The synthetic route of the complex is shown in Fig.2.
A solution of copper(Ⅱ) acetate monohydrate (18.98 mg,0.10 mmol)in ethanol(8 mL)was added dropwise to a solution of H4L (33.3 mg,0.10 mmol)in acetone(10 mL)at room temperature,and a dark-green solution was obtained immediately.The solution was placed in a hexane atmosphere,about several weeks later,along with diffusion of hexane into the mixed solution of the complex,the single crystals suitable for X-ray crystallographic analysis were obtained.Anal.Calcd.for C36H38Cu3N4O14{[Cu(HL)(EtOH)]2Cu}(%):C, 45.93;H,4.07;N,5.95;Cu,20.25.Found(%):C, 45.80;H,4.05;N,5.98;Cu,20.32.

1.4 Crystal structure determination
The single crystal of the complex with approximate dimensions of 0.40 mm×0.10 mm×0.05 mm was placed on a Bruker Smart 1000 CCD diffractmeter.The diffraction data were collected using a graphite monochromated Mo Kα radiation (λ=0.071073 nm)at 298(2)K.The Lp factor and Semi-empirical absorption corrections were applied to the intensity data.The structure was solved by using the program SHELXS-97[15]and difference Fourier techniques,and refined by full-matrix least-squares method on F2using SHELXL-97[16].The non-hydrogen atoms were refined anisotropically.Hydrogen atoms were added theoretically.The data collection and refinements of CuⅡcomplex is given in Table 1.
CCDC:811856.

Table 1 Crystal data and structure refinement for CuⅡcomplex
2 Results and discussion
2.1 FTIR spectra
Main IR data of CuⅡcomplex is given in Table 2.IR spectra of H2L and its complex exhibit several distinguishableresonancesin the 400~4000 cm-1region consistent with the coordination geometry as revealed from structure determination.
The O-H stretching frequency of H2salen-type ligand is expected in the 3 300~3 800 cm-1region,but this frequency is displaced by 3 425 cm-1for H4L because of the internal hydrogen bond OH…N=C[17].For the CuⅡcomplex,the appearance of this band is expected due to the existence of hydroxy group.The characteristic C=N stretching band of the free ligand H4L appears at 1 612 cm-1,whereas the C=N band of the CuⅡcomplex is observed at 1 605 cm-1.The Ar-O stretching frequencies appear within 1 263~1 213 cm-1as reported for similar H2salen-type ligands[2,18].These bands occur at 1 266 cm-1for H4L,and 1 260 cm-1for the complex.The weaker bands at 460 and 421 cm-1for the CuⅡcomplex are assigned to ν(Cu-N)and ν(Cu-O), respectively[2-3,5].These bands are observed in the complex as new bands,and are not present in the spectrum of the free ligand H4L.The metal-oxygen and metal-nitrogen frequency assignments pointed out by Percy and Thorntonare very difficult at times[19].
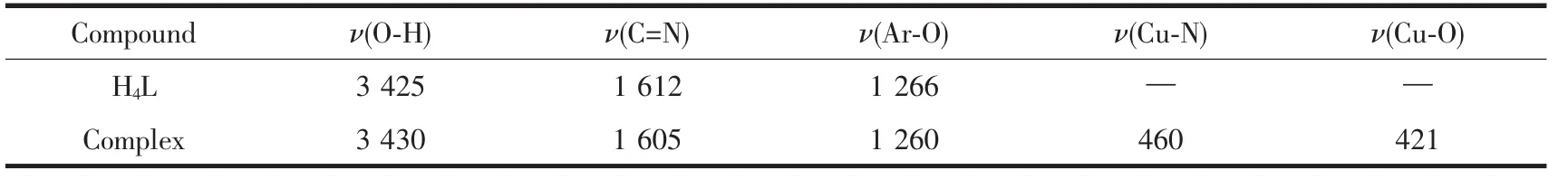
Table 2 Main IR bands for H4L and its CuⅡcomplex (cm-1)
2.2 Molar conductance
The complex is soluble in DMF,DMSO,but not soluble in ethanol,methanol,acetonitrile,acetone, THF,ethyl acetate and hexane.Molar conductance values of the CuⅡcomplex at 25℃of 1.0 mmol·dm-3DMF solutions is 19.8 Ω-1·cm2·mol-1,indicating that the CuⅡcomplex is non-electrolyte.This implies that all the(HL)3-units in the CuⅡcomplex are always held in the coordination sphere in solution or solid state.
2.3 Thermal property
The results show the TG curve of the complex can be divided into two stages.The first stage starts from 79 to 96℃ with the weight loss of 10.1%,which corresponds to the loss of two ethanol molecules (theoretical mass loss,9.8%)which takes part in coordination to the Cu(Ⅱ)ions.The solid remains stable up to 203℃and the second weight loss starts at around 212℃with decomposition of the compound.The TG curve shows around 76.5%total mass loss at 800℃ indicating the complete removal of the(HL)3-units.The main residual product was CuO with the value of 22.5% (theoretical residual value was 25.4%).
2.4 Crystal structure of the CuⅡcomplex


Table 3 Bond lengths(nm)and bond angles(°)for the CuⅡcomplex
X-ray crystallographic analysis reveals the crystal structure of the complex.Selected bond lengths and angles are summarized in Table 3.
The coordination environment of the Cu(Ⅱ)ions are shown in Fig.3.The complex crystallizes in the monoclinic system,space group C2/c,which consists of three CuⅡions,two pentadentate(HL)3-units,two coordinated EtOH molecules.The CuⅡcomplex possessing two five-coordinated CuⅡand one four-coordinated CuⅡions,the former CuⅡ(Cu1 and Cu1#1)centres are linked by two nitrogen (N1 and N2)atoms and two phenoxo oxygen (O1 and O5)atoms of one pentadentate(HL)3-trianion and one oxygen(O7)atom from one coordinated EtOH molecule,and the later CuⅡ(Cu2)centre is fourcoordinated by two phenoxo oxygen(O1 and O2)atoms of one pentadentate (HL)3-trianion and two phenoxo oxygen (O1#1and O2#1)atoms of another pentadentate (HL)3-trianion.
The two penta-coordinated CuⅡions of the complex have a slightly distorted tetragonal pyramidal coordinated polyhedron.The two Cu1 and Cu1#1ions are located in the N2O2coordination sphere of(HL)3-unit.The O7 atom from the coordinated EtOH molecule is also coordinated to Cu1 ion.Consequently,the dihedral angle between the coordination plane of O1-Cu1-N1 and that of O5-Cu1-N2 is about 15.33(2)°, indicating slight distortion from the square planar structure,which is same as the environment of the other Cu complex[20].
The basal face of Cu1 is defined by O1,O5,N1 and N2 atoms,deviation of Cu1 0.177(2)nm,the apical position is occupied by O7 atom of the EtOH molecule. Whereas the basal face of Cu2 is defined by O1,O2, O1#1,O2#1atoms deviation of Cu2 0.117(2)nm.So the two CuⅡ(Cu1 and Cu1#1)ions are five-coordinated,and aother CuⅡ(Cu2)ion is four-coordinated.The trinuclear structure is probably stabilized by the two (HL)3-ligands,which neutralize the whole charge of CuⅡcomplex.
The Cu1…·Cu2 or Cu2…Cu1#1intramolecular separation is 0.352 3(3)nm,which is not sufficiently short to imply metal-metal bonding interaction.The bridging angle of Cu1-O1-Cu2 is 126.42(3)°,indicating non-collinear Cu-O-Cu fragments,not suitable for significant electronic exchange[21].
As shown in Table 4,intermolecular C10-H9…O6 hydrogen bond linked neighboring two molecules. And each deprotonated O2 atoms of the (HL)3-unit is hydrogen-bonded to the-O6-H13 groups of the complex molecule and-O7-H14 groups of coordinating EtOH molecule,whereas the-C10-H9 groups are hydrogenbonded to undeprotonated O6 atom of the(HL)3-unit of another complex molecule.The crystal packing of the complex shows that a notable feature of this structure resides in the formation of a new 2D hydrogen-bonding supramolecular networks through intermolecular C-H…O interactions.

Table 4 Hydrogen bonding distances and bond angles for the CuⅡcomplex
[1]Costamagna J,Vargas J,Latorre R,et al.Coord.Chem.Rev., 1992,119:67-68
[2]Dong W K,Sun Y X,Zhang Y P,et al.Inorg.Chim.Acta, 2009,362(1):117-124
[3]Dong W K,Shi J Y,Xu L,et al.Appl.Organometal.Chem., 2008,22(2):89-96
[4]Canali L,Sherrington D C.Chem.Soc.Rev.,1999,28:85-93
[5]DONG Wen-Kui(董文魁),Lü Zhong-Wu(呂忠武),SUN Yin-Xia(孫銀霞),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(9):1627-1634
[6]DONG Wen-Kui(董文魁),TANG Xiao-Lu(唐曉璐),HE Xue-Ni(何雪妮),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(3):528-532
[7]Zhu H L,Tong Y X,Chen X M,et al.Trans.Met.Chem., 2001,26:528-531
[8]Dong W K,Feng J H,Yang X Q.Synth.React.Inorg.Met-Org. Nano-Met.Chem.,2007,37(3):189-192
[9]XU Li(許力),ZHANG Yan-Ping(張艷萍),SUN Yin-Xia(孫銀霞),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2007,23(11):1999-2002
[10]Sharma A K,Lloret F,Mukherjee R.Inorg.Chem.,2007,46: 5128-5130
[11]XU Guo-Jin(徐國津),TANG Yu-Hai(唐玉海),WEI Sai-Li(魏賽麗),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2009,25(8):1359-1365
[12]XIANG Ping(項萍),CHEN Long-Hai(陳龍海),WU Jin-Cai (鄔金才),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(3):391-396
[13]Dong W K,Shi J Y,Zhong J K,et al.Struct.Chem.,2008,19: 95-99
[14]DONG Wen-Kui(董文魁),SHI Jun-Yan(史軍妍),ZHONG Jin-Kui(鐘金魁),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(1):10-14
[15]Sheldrick G M.SHELXS97,Program for the Solution of Crystal Structures,University of G?ttingen,Germany,1997.
[16]Sheldrick G M.SHELXL97,Program for the Refinement of Crystal Structures,University of G?ttingen,Germany,1997.
[17]Dong W K,Duan J G,Guan Y H,et al.Inorg.Chim.Acta, 2009,362(4):1129-1134
[18]Asadi M,Jamshid K A,Kyanfar A H.Inorg.Chim.Acta, 2007,360(5):1725-1730
[19]Percy G C,Thornton J.J.Inorg.Nucl.Chem.,1973,35:2319-2327
[20]Dong W K,Feng J H,Wang L,et al.Trans.Met.Chem., 2007,32(8):1101-1105
[21]Gerli A,Hagen K S,Marzilli L G.Inorg.Chem.,1991,30: 4673-4676
Synthesis and Structure of Trinuclear CuⅡComplex{[Cu(HL)(EtOH)]2Cu}
DING Yu-Jie*,1WANG Yang2
(1College of Biochemical Engineering,Anhui Polytechnic University,Wuhu,Anhui 241000,China)
(2Material Examination Department,State Intellectual Property Office of the P.R.C.,Beijing 100088,China)
A new CuⅡcomplex,{[Cu(HL)(EtOH)]2Cu}(H4L=6,6′-dihydroxy-2,2′-[(1,2-ethylenedioxybis(nitrilomethylidyne)]diphenol),has been synthesized and characterized by elemental analysis,IR spectroscopy,and X-ray single crystal diffraction method.The results show that the complex consists of three CuⅡions,two pentadentate (HL)3-units,two coordinated EtOH molecules.The two penta-coordinated CuⅡions of the complex have a slightly distorted tetragonal pyramidal geometry,and the other tetra-coordinated CuⅡion of the complex has a slightly square planar geometry.The crystal packing of the complex shows that a notable feature of this structure resides in the formation of an infinite 2D supramolecular networks through intermolecular C-H…O interactions.CCDC:811856.
bisoxime ligand;CuⅡcomplex;synthesis;crystal structure
O614.81+2
A
1001-4861(2011)07-1411-06
2011-02-18。收修改稿日期:2011-04-20。
安徽省高等學校優(yōu)秀青年人才基金(No.2011SQRL075)資助項目。
*通訊聯系人。E-mail:dyj@ahpu.edu.cn;會員登記號:S06N4260M1005。

