Influence of Accelerated Aging on Detonation Performance of Explosives
GAO Da-yuan(高大元),HUA Cheng(花成),WANG Xiang(王翔),HAN Yong(韓勇)
(Institute of Chemical Materials,China Academy of Engineering Physics,Mianyang 621900 Sichuan,China)
Introduction
The detonation performance is one of important characteristics for explosives,mostly measured by experiments and calculated by experiential formula and state equation[1-2]. During the accelerated aging,some slow thermal decomposition and composition transplants occur in the explosive,also,some defects difficult to be observed by eyes would be produced.The density and composition changes of explosives result in the detonation performance change.Many researchers try to use the numerical simulation to study the influence of accelerated aging on the detonation performance of explosives.To calculate the detonation parameters of explosives after partial thermal decomposition accurately,the characteristics of thermal decomposition,accelerated aging mechanism and aging effect of the explosive under different temperatures should be known.In this paper,the kinetics parameters,mechanism function and kinetic equation of the thermal decomposition are obtained from the thermo-gravimetric(TG)curves of GI-1,PBX-1 and PBX-2 explosives at different heat rates,then,the change in density,composition and heat of formation of the explosives are calculated in the accelerated aging,the change of detonation parameters of GI-1,PBX-1 and PBX-2 are also calculated using VLWR code.
1 Research of Thermal Decomposition Kinetics
For the thermal decomposition of explosives under constant heat rate,while the kinetics equation is studied by non-isothermal method,the relationship between reaction rate constantkand temperatureTcan be expressed by Arrhenius equation.Then,Ozawa's equation can be derived theoretically,and given as[3-4]
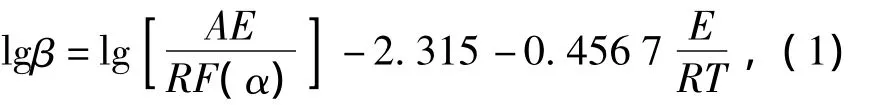
whereβis the heat rate,K·min-1,αthe decomposition extent of explosives,%,F(xiàn)(α)the integral formula of mechanism function,Athe pre-exponential factor,s-1,Ethe activation energy,J·mol-1,Rthe ideal gas constant,J·mol-1·K-1,Tthe temperature,K.In the sameα,logβrelates to 1/Tlinearly.The slope of the linear represents the activation energy,and it can be used to conclude the mechanism function of thermal decomposition.Based on the Doyle's method,Eq.(1)may be transformed as
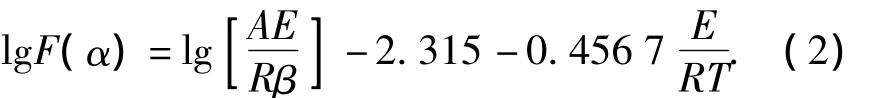
From Eq.(2),it can be seen that lgF(α)re-lates to 1/Tlinearly also for any mechanism of thermal decomposition.For a certain reaction mechanism function,if the activation energy obtained by Doyle's method is very close to the activation energy obtained by Ozawa's method,then it can be determined as the mechanism function of thermal decomposition[5].
In our experiments,the thermal decomposition apparatus is NETZSCH STA 449C DSC-TG made in Germany,the sample weight is 5.00 mg,the temperature ranges in 20℃ ~500℃.The TG curves of GI-1,PBX-1 and PBX-2 explosives at different heat rates are given in Fig.1,2 and 3 respectively.
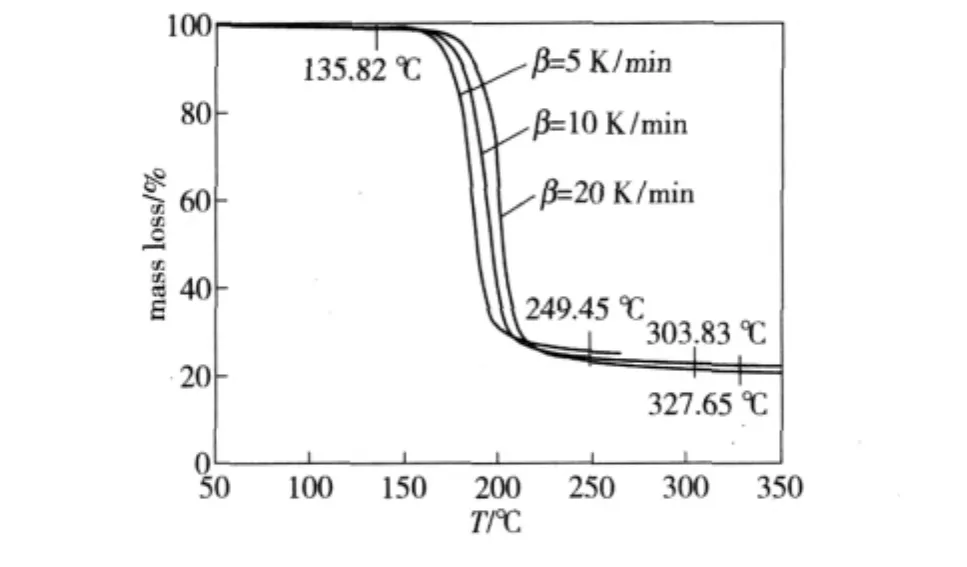
Fig.1 TG curves of GI-1 explosive at different heat rates
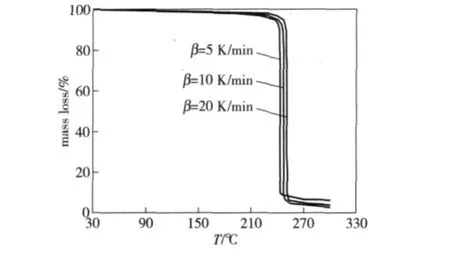
Fig.2 TG curves of PBX-1 explosive at different heat rates

Fig.3 TG curves of PBX-2 explosive at different heat rates
For GI-1,PBX-1 and PBX-2 explosives,the reaction temperaturesTcorresponding to various decomposition extentsαare obtained from TG curves of explosives under the heat rate of 5,10 and 20 K·min-1,respectively,and the kinetics parameters and mechanism functions of the thermal decomposition reaction are calculated by Ozawa's method and Doyle's method,the results are listed in Table 1.
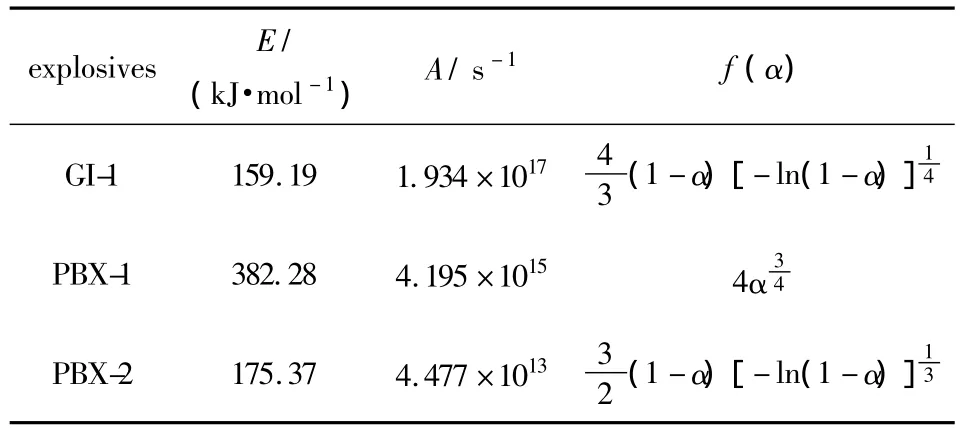
Table 1 Kinetics parameters and mechanism functions of thermal decomposition reaction of explosives
The kinetics equations of the thermal decomposition for three explosives are
GI-1:
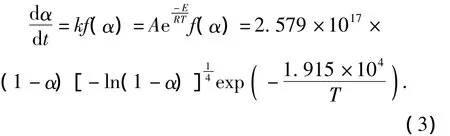
PBX-1:
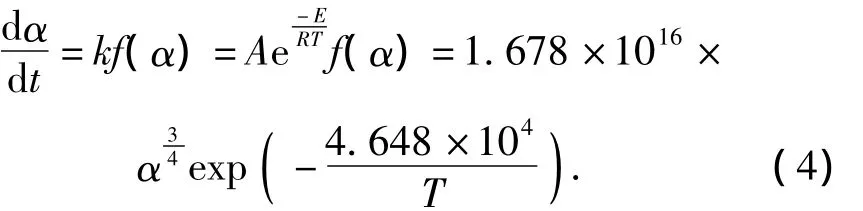
PBX-2:
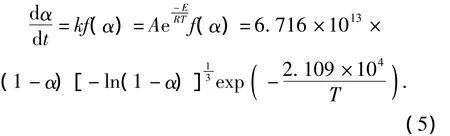
Based on the mechanism function and kinetic equations of thermal decomposition,the changes of mass fraction on various decomposition extentsαunder different accelerated aging temperatures can be calculated,and then the changes of density,composition and heat of formation can be found out.
2 Influence of Accelerated Aging on Detonation Performance
The change of density,composition and heat of formation for GI-1,PBX-1 and PBX-2 explosives in accelerated aging can be calculated respectively using Eq.(6)
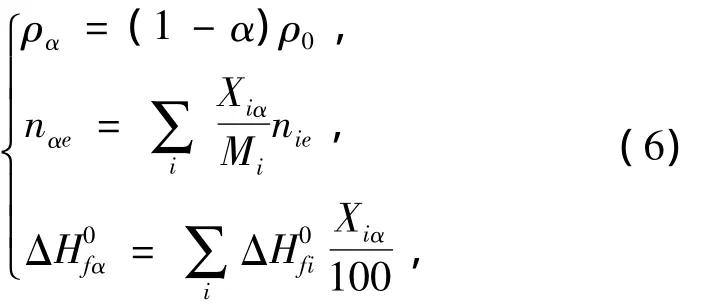
whereρ0is the density of novel explosives,g·cm-3,ραthe density of explosives on the decomposition extent ofα,g·cm-3,Mithe molar mass of ingredientiin explosives formula,g·mol-1,Xiαthe percentage of ingredientiof explosives on the decomposition extent ofα,erepresents C,H,N,O,F(xiàn),Cl and Si elements of explosives respectively,nieare the mole number of C,H,N,O,F(xiàn),Cl and Si element in ingredienti,nαeis the mole number of C,H,N,O,F(xiàn),Cl and Si element in explosives on the decomposition extent ofα,Δis the heat of formation of ingredientiin explosives,kJ·mol-1,Δis the heat of formation of explosives on the decomposition extent ofα,kJ·mol-1.
The initial parameters of GI-1,PBX-1 and PBX-2 explosive cylinders are listed in Table 2.The detonation velocities and pressures can be calculated using VLWR code[6-8]on the decomposition extents of 3% ,2%,2%for GI-1,PBX-1 and PBX-2 explosives under accelerated aging temperatures 70℃and 75℃,compared with the detonation parameters of the original explosives,the results are given in Fig.4,5 and 6,respectively.
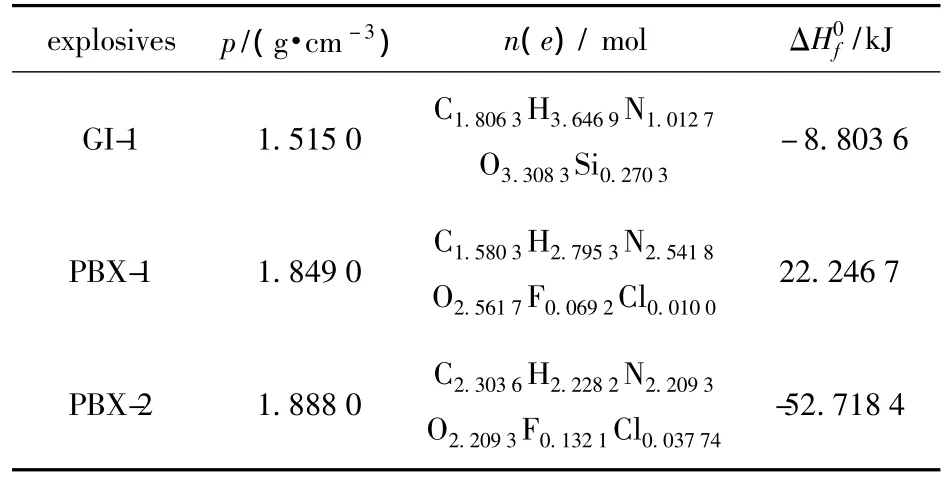
Table 2 Initial parameters of GI-1,PBX-1 and PBX-2 explosives
Figure 4 shows that CJ velocity and pressure of GI-1 explosive are 7.273 mm·μs-1and 20.08 GPa respectively.If the decomposition extent is 3%,the density changes to 1.4696 g·cm-3and decreases about 0.045 4 g·cm-3,the CJ velocity changes to 7.087 mm·μs-1and decreases about 0.186 mm·μs-1,and the CJ pressure changes to 18.59 GPa and decreases about 1.49 GPa.The aging takes 559.50 days under temperatures of 70℃,and the aging takes 250.88 days under temperatures of 75℃.
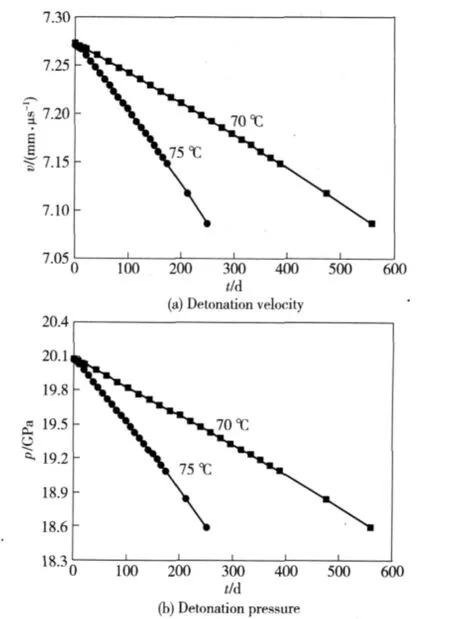
Fig.4 Changes of detonation velocity and pressure of GI-1 explosive under accelerated aging temperatures of 70℃and 75℃
Figure 5 shows that CJ velocity and pressure of PBX-1 explosive are 8.691 mm·μs-1and 34.39 GPa respectively under initial condition.If the decomposition extent is 2%,the aging time takes 2 596.88 days under temperatures 70℃,the density changes to 1.803 0 g·cm-3and decreases about 0.046 g·cm-3,the CJ velocity changes to 8.534 mm·μs-1and decreases 0.157 mm·μs-1,and CJ pressure changes to 32.14 GPa and decreases about 2.25 GPa.While under aging temperatures of 75℃,it needs 1 257.71 days to decomposition to the extent of 2%,the density changes to 1.804 5 g·cm-3and decreases about 0.044 5 g·cm-3,CJ velocity changes to 8.539 mm·μs-1and decreases about 0.152 mm·μs-1,and CJ pressure changes to 32.21 GPa and decreases about 2.18 GPa.
Figure 6 shows that CJ velocity and pressure of PBX-2 explosive are 7.656 mm·μs-1and 23.60 GPa respectively under initial condition.If the decomposition extent is 2%,the density changes to 1.850 2 g·cm-3and which decreases about 0.037 8 g·cm-3,the CJ velocity changes to 7.513 mm·μs-1and decrea-ses about 0.143 mm·μs-1,and CJ pressure changes to 22.53 GPa and decreases about 1.07 GPa.The aging takes 10 777.49 days under temperature of 70℃and only 4 423.76 days under temperature of 75℃.
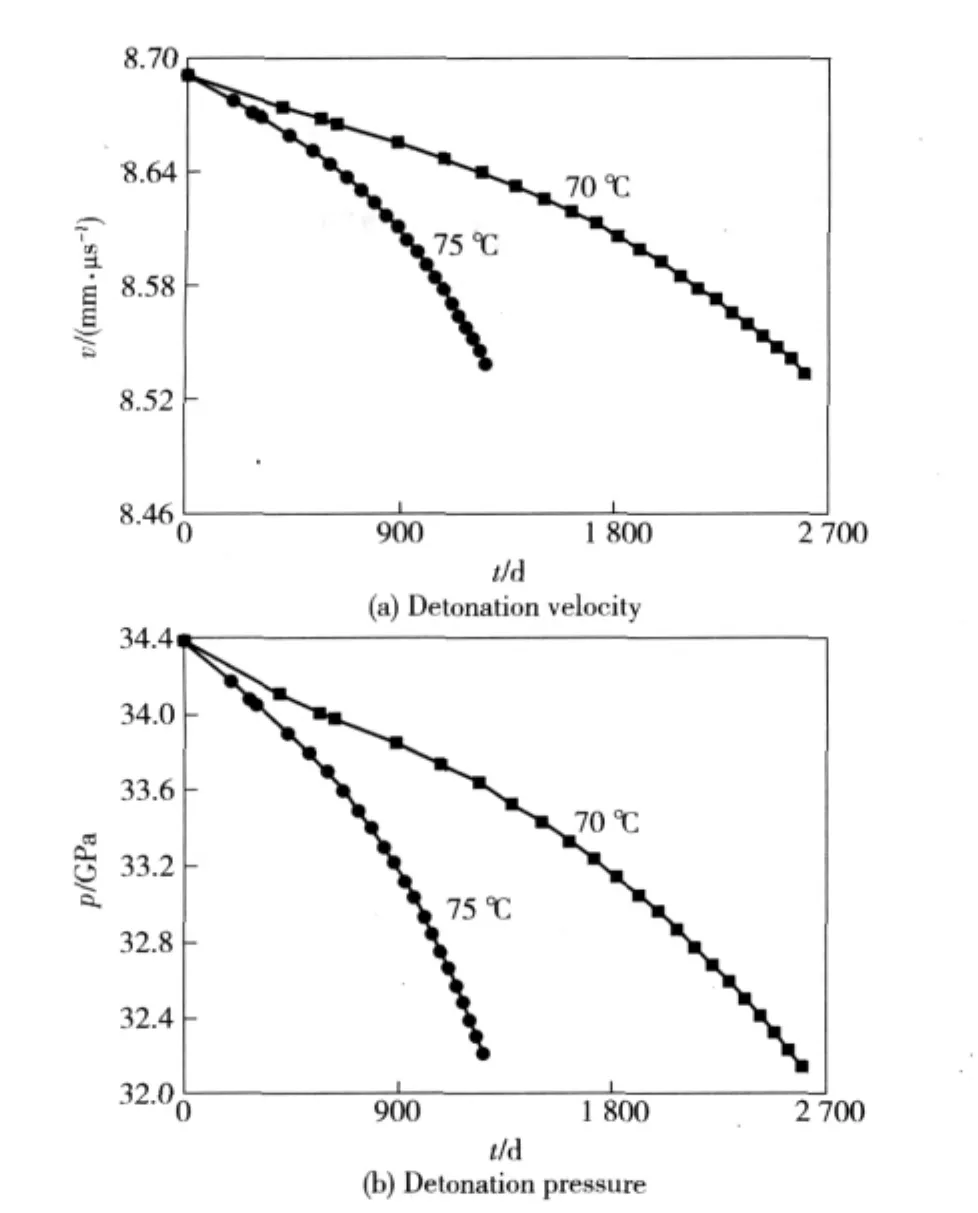
Fig.5 Changes of detonation velocity and pressure of PBX-1 explosive under accelerated aging temperatures of 70℃and 75℃
3 Conclusions

Fig.6 Changes of detonation velocity and pressure of PBX-2 explosive under accelerated aging temperatures of 70℃and 75℃
1)Based on TG curves of different explosives under different heat rates,the activation energy,pre-exponential factor,mechanism function and kinetics equation of the thermal decomposition can be obtained using Ozawa and Doyle methods.
2)Based on the kinetics equation of the thermal decomposition and mixed theorem,the changes of the density,composition and heat of formation on various decomposition extents of GI-1,PBX-1 and PBX-2 explosives can be calculated respectively in accelerated aging.The changes of the detonation parameters for explosives can be calculated further by means of the VLWR code.
3)After accelerated aging,the explosive density is decrease,the detonation velocity and detonation pressure are all decreased slightly.
[1]DONG Hai-shan,ZHOU Fen-fen.Performance of high explosives and correlates[M].Beijing:Science Press,1989.(in Chinese)
[2]CHU Shi-jin.Thermal analysis of explosives[M].Beijing:Science Press,1994.(in Chinese)
[3]HU Rong-zu,GAO Sheng-li.Kinetics of thermal analysis[M].Beijing:Science Press,2007.(in Chinese)
[4]Ozawa T.A new method of analyzing thermogravimetric data[J].Bulletin of Chemical Society of Japan,1965,38(11):1881-1886.
[5]GAO Da-yuan,DONG Hai-shan,LI Bo-tao.Research and application of thermal decomposition kinetics for explosives[J].Chinese Journal of Energetic Materials(Supplement),2004:307-310.(in Chinese)
[6]WU Xiong,LONG Xin-ping.Review and look forward to the progress of VLW equation of state[J].Chinses Journal of High Pressure Physics,1999,13(1):55 -58.(in Chinese)
[7]GAO Da-yuan,XU Rong.Detonation performance of TATB,TCTNB and TCDNB[J].Chinses Journal of Explosives &Propellants,2005,28(2):68-71.(in Chinese)
[8]GAO Da-yuan.Experimental and theoretical study on detonation and safety for composted explosives[D].Nanjing:Nanjing University of Science and Technology,2003.(in Chinese)
- Defence Technology的其它文章
- Analysis on Velocity Characteristics of Cavitation Flow Around Hydrofoil
- Research on Additional Loss of Guidance Optical Fiber
- An HLA/RTI Architecture Based on Multi-thread Processing
- A Fuzzy Adaptive Algorithm Based on“Current”Statistical Model for Maneuvering Target Tracking
- Quantitative Analysis of Components in OC-CS Sprays by High Performance Liquid Chromatography with Double Wavelength UV Detection
- A New Chaotic Genetic Hybrid Algorithm and Its Applications in Mechanical Optimization Design

