Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
SUN Zihong (孫紫紅) and YUAN Anbao (袁安保)**
?
Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
SUN Zihong (孫紫紅) and YUAN Anbao (袁安保)**
Department of Chemistry, College of Sciences, Shanghai University, Shanghai 200444, China
Polyvinyl alcohol (PVA)-sodium polyacrylate (PAAS)-KOH-H2O alkaline polymer electrolyte film with high ionic conductivity was prepared by a solution-casting method. Polymer Ni(OH)2/activated carbon (AC) hybrid supercapacitors with different electrode active material mass ratios (positive to negative) were fabricated using this alkaline polymer electrolyte, nickel hydroxide positive electrodes, and AC negative electrodes. Galvanostatic charge/ discharge and electrochemical impedance spectroscopy (EIS) methods were used to study the electrochemical performance of the capacitors, such as charge/discharge specific capacitance, rate charge/discharge ability, and charge/discharge cyclic stability. Experimental results showed that with the decreasing of active material mass ratio(Ni(OH)2)/(AC), the charge/discharge specific capacitance increases, but the rate charge/discharge ability and the charge/discharge cyclic stability decrease.
PVA based alkaline polymer electrolyte, Ni(OH)2/AC supercapacitor, electrode active material mass ratio, electrochemical performance
1 INTRODUCTION
As a charge storage device, electrochemical supercapacitor has the advantages of higher energy density than conventional electrostatic capacitor and higher power density than commonly used rechargeable battery, as well as long cycle life. Therefore, supercapacitors attracted great attentions in recent years for possible use in consumer electronics as power sources and in electric vehicles as auxiliary power systems. Compared with supercapacitors with liquid electrolytes, there have been fewer researches about supercapacitors with polymer or gel electrolytes [1-17]. Park. reported a hybrid supercapacitor using Ni(OH)2/activated carbon (AC) composite positive electrode, AC negative electrode, and 6 mol·L-1KOH electrolyte [18]. The specific capacitance of the hybrid capacitor (HC) was found to be far higher than that of the symmetrical AC/AC electric double layer capacitor (EDLC) owing to the high faradic specific pseudocapacitance of Ni(OH)2. Recently, Nohara. reported a polymerNi(OH)2/AC hybrid supercapacitor using a cross-linkedpotassium polyacrylate (PAAK) based PAAK-KOH-H2Opolymer hydrogel electrolyte [15]. This HC was charge/ discharge tested in the voltage range of 0.4-1.2 V. The capacitance and high-rate dischargeablity were found to be superior to that of the symmetrical AC/AC capacitor, and a good charge/discharge cyclic stability was achieved. However, the electrode active material mass ratio and specific capacitance data for the capacitor were not mentioned in the article.
Electrochemical devices with polymer electrolytes have the particular advantages of leak-free, flexible, and safety. In fact, polymer electrolytes have been successfully applied to polymer lithium ion batteries and polymer electrolyte membrane fuel cells. Alkaline KOH solution is a well-known electrolyte, which can be used in various electrochemical systems, such as batteries with K2FeO4or NiOOH cathode [19, 20]. Recently, alkaline polymer electrolytes, especially the polyvinyl alcohol (PVA) based alkaline polymer electrolytes, have attracted increasing attentions owing to their high ionic conductivity [14, 21-37]. However, we found that PVA based alkaline polymer electrolytes tend to lose water profoundly in atmosphere, resulting in reduction of conductivity and mechanical property [33]. In order to overcome this drawback, small amount of hydrophilic sodium polyacrylate (PAAS) was added to PVA for modification, and a novel PVA-PAAS-KOH-H2O type PVA based alkaline polymer electrolyte film with a high room-temperature ionic conductivity of 9.67×10-2S·cm-1was prepared in our previous work [37]. In the present work, the polymer Ni(OH)2/AC hybrid supercapacitors using this alkaline polymer electrolyte were fabricated, and the influence of electrode active material mass ratio on their electrochemical performance were investigated.
2 EXPERIMENTAL
2.1 Preparation and conductivity determination of alkaline polymer electrolyte film
Alkaline polymer electrolyte film was prepared using a solution-casting method. Given amount of PVA and PAAS were dissolved, respectively, in distilled water and magnetically stirred for several hours. The two solutions were then mixed, and a definite amount of KOH solution was added to the mixture. The mixture was stirred again for some time, and finally, a homogeneous solution was obtained. The solution was poured onto a clean glass plate for evaporation of excessive water in air atmosphere, and then the alkaline polymer electrolyte film was obtained. The thickness of the film is ca. 0.5 mm with a composition of PVA (13.84%, by mass until specified otherwise)-PAAS (0.73%)-KOH (36.43%)-H2O (49%). The addition of PAAS to PVA in the present work is aimed to improve the water retention capacity of the PVA based alkaline polymer electrolyte film, and hence, to make the electrolyte film flexible with a higher ionic conductivity.

2.2 Fabrication of polymer Ni(OH)2/AC supercapacitors
Nickel hydroxide positive electrode was prepared by mixing a given amount of Ni(OH)2with 5% cobalt powder and 2% acetylene black, then some polytetrafluoroethylene (PTFE) emulsion was added to the mixture to form slurry. The slurry was filled into a foamed nickel with an apparent area of 2 cm×2 cm, dried at 65°C, and rolled to a sheet. Activated carbon negative electrode was prepared by mixing a given amount of AC and acetylene black with a mass ratio 85︰15, and the remaining procedure was the same as the procedure for the preparation of Ni(OH)2electrode. The liquid Ni(OH)2/AC capacitor consisted of a Ni(OH)2positive electrode and an AC negative electrode separated by a polypropylene separator and 6 mol·L-1KOH solution as electrolyte. It was charge/discharge cycled for three times at a lower current density (0.1C rate for the Ni(OH)2electrode) for activation. The activated Ni(OH)2electrode and AC electrode were then separated and dried naturally in air atmosphere. The polymer Ni(OH)2/AC capacitors were fabricated using the PVA based alkaline polymer electrolyte film sandwiched by the activated Ni(OH)2and AC electrodes. In order to make the electrodes contact well with the polymer electrolyte film, the fabricated polymer capacitors were rolled again to an appropriate thickness.
2.3 Electrochemical testing of polymer Ni(OH)2/AC supercapacitors
Electrochemical impedance spectroscopy (EIS) measurements for the liquid and polymer Ni(OH)2/AC capacitors were carried out in the frequency range of 10-2-106Hz, using a Solartron 1287 electrochemical interface coupled with a 1255B FRA. Galvanostatic charge/discharge tests of the polymer capacitors were performed using a LAND auto-cycler (Wuhan, China) in the voltage range of 0.3-1.5 V. All the work was carried out at room temperature.
The charge/discharge current density and specific capacitance of the polymer capacitors in the present work were calculated according to the following Eqs. (1) and (2), respectively:
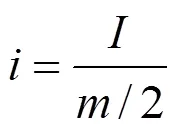

whereis the charge/discharge current density,is the charge/discharge current,is the total mass of the active materials of positive and negative electrodes,sis the specific capacitance of the capacitor,is the discharge time, andDis the operating voltage range.
3 RESULTS AND DISCUSSION
3.1 Electrochemical impedance spectroscopy study


Figure 1 Electrochemical impedance spectroscopy measurements
● liquid; ○ polymer
3.2 Charge/discharge curves of polymer capacitors
Figure 2 shows the charge/discharge curves of the polymer capacitors with different active material mass ratios at a current density of 300 mA·g-1. The observed charge and discharge curves, herein, are curved somewhat with a fundamental symmetry, which are similar to the observations reported in literatures [15, 38]. This suggests a faradic pseudocapacitive characteristic of the capacitors [38], which is different from that of a typical capacitor. At the same charge/discharge current density, the charge/discharge durations for the three capacitors are quite different. With increasing mass ratio(Ni(OH)2)/(AC), the charge/discharge duration (in other words, the specific capacity or specific capacitance) decreases. This is because the specific capacitance of the Ni(OH)2electrode (faradic pseudocapacitance) is far larger than that of the AC electrode (EDLC capacitance). For every capacitor, the capacity of the positive electrode exceeds that of the negative electrode,.., the AC negative electrode governs the capacity of the capacitor. With the increase in mass ratio, the relative amount of AC in the capacitor decreases. Thus, on the one hand, the specific capacitance of the capacitor should decrease. On the other hand, the actual applied current density of the AC electrode should increase, resulting in increase of electrode polarization. Hence, with the increase in mass ratio, the charge/discharge duration or specific capacitance decreases.
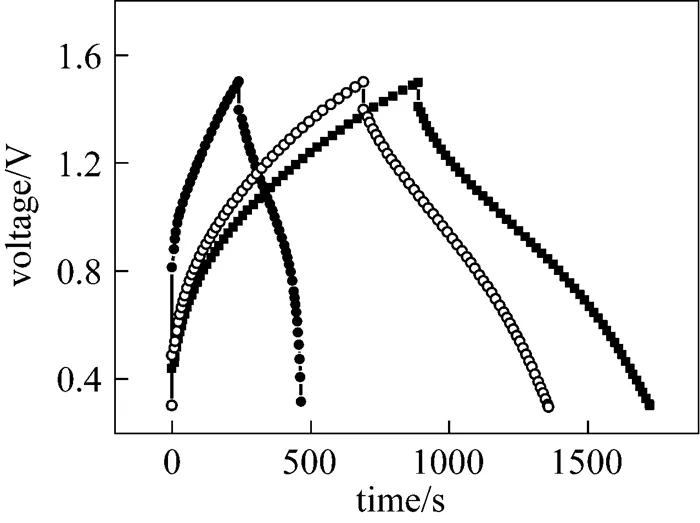
Figure 2 Charge/discharge profiles of the polymer i(OH)2/AC capacitors with different mass ratios at a current density of 300 mA·g-1
3.3 Rate dischargeability of polymer capacitors


Figure 3 Variation of discharge specific capacitance with charge/discharge current density for the polymer Ni(OH)2/AC capacitors with different active material mass ratios
3.4 Cycle lives of polymer Ni(OH)2/AC capacitors


Figure 4 Charge/discharge cycle lives of the polymer Ni(OH)2/AC capacitors with different active material mass ratios at a current density of 300 mA·g-1

Figure 5 Nyquist plots of the impedance for the polymer Ni(OH)2/AC capacitor before and after long-term charge/ discharge cycling and the close-up view of the left plot in high-frequency region
●?before cycling;○?after cycling

4 CONCLUSIONS
Polyvinyl alcohol (13.84%)-PAAS (0.73%)-KOH (36.43%)-H2O (49%) alkaline polymer electrolyte filmwas prepared by a solution-casting method. This polymer electrolyte showed a high ionic conductivity of ca. 0.1 S·cm-1at room temperature. Polymer Ni(OH)2/AC hybrid electrochemical capacitors were fabricated using this alkaline polymer electrolyte, Ni(OH)2positive electrode, and AC negative electrode. Electrochemical impedance spectroscopy results indicated that the fabricated polymer capacitor shows a good interfacial compatibility at the electrode/polymer electrolyte interface. This polymer electrolyte film can be used in Ni(OH)2/AC capacitors. Galvanostatic charge/discharge results demonstrated that with decrease in electrode active material mass ratio, the specific capacitance of the polymer Ni(OH)2/AC capacitor increases, but the rate dischargeability and charge/discharge cycleability decreases. In other words, the electrode active material mass ratio has a crucial influence on electrochemical performance of the capacitor. Hence, we should consider simultaneously the specific capacitance, rate dischargeability, and long-term charge/discharge cycleability when designing a polymer Ni(OH)2/AC hybrid supercapacitor.
1 Lassègues, J.C., Grondin, J., Becker, T., Servant, L., Hernandez, M., “Supercapacitor using a proton conducting polymer electrolyte”,, 77, 311-317(1995).
2 Panero, S., Clemente, A., Spila, E., “Solid state supercapacitors using gel membranes as electrolytes”,, 86-88, 1285-1289 (1996).
3 Osaka, T., Liu, X., Nojima, M., Momma, T., “An electrochemical double layer capacitor using an activated carbon electrode with gel electrolyte binder”,..., 146, 1724-1729 (1999).
4 Gu, H.B., Kim, J.U., Song, H.W., Park, G.C., Park, B.K., “Electrochemical properties of carbon composite electrode with polymer electrolyte for electric double-layer capacitor”,., 45, 1533-1536 (2000).
5 Chojnacka, J., Acosta, J.L., Morales, E., “New gel electrolytes for batteries and supercapacitor applications”,., 97-98, 819-821(2001).
6 Lewandowski, A., Zajder, M., Frackowiak, E., Béguin, F., “Supercapacitor based on activated carbon and polyethylene oxide-KOH-H2O polymer electrolyte”,., 46, 2777-2780 (2001).
7 Erokhin, V., Raviele, G., Glatz-Reichenbach, J., Narizzano, R., Stagni, S., Nicolini, C., “High-value organic capacitor”,..., 22, 381-385 (2002).
8 Hashmi, S.A., Upadhyaya, H.M., “Polypyrrole and poly(3-methyl thiophene)-based solid state redox supercapacitors using ion conducting polymer electrolyte”,, 152/153, 883-889 (2002).
9 Latham, R.J., Rowlands, S.E., Schlindwein, W.S., “Supercapacitors using polymer electrolytes based on poly(urethane)”,, 147, 243-248 (2002).
10 Nohara, S., Wada, H., Furukawa, N., Inoue, H., Morita, M., Iwakura, C., “Electrochemical characterization of new electric double layer capacitor with polymer hydrogel electrolyte”,., 48, 749-753 (2003).
11 Sung, J.H., Kim, S.J., Lee, K.H., “Fabrication of all-solid-state electrochemical microcapacitors”,., 133, 312-319 (2004).
12 Lufrano, F., Staiti, P., “Performance improvement of Nafion based solid state electrochemical supercapacitor”,., 49, 2683-2689 (2004).
13 Wada, H., Nohara, S., Furukawa, N., Inoue, H., Sugoh, N., Iwasaki, H., Morita, M., Iwakura, C., “Electrochemical characteristics of electric double layer capacitor using sulfonated polypropylene separator impregnated with polymer hydrogel electrolyte”,., 49, 4871-4875 (2004).
14 Yang, C.C., Hsu, S.T., Chien, W.C., “All solid-state electric double-layer capacitors based on alkaline polyvinyl alcohol polymer electrolytes”,., 152, 303-310 (2005).
15 Nohara, S., Asahina, T., Wada, H., Furukawa, N., Inoue, H., Sugoh, N., Iwasaki, H., Iwakura, C., “Hybrid capacitor with activated carbon electrode, Ni(OH)2electrode and polymer hydrogel electrolyte”,., 157, 605-609 (2006).
16 Kalpana, D., Renganathan, N.G., Pitchumani, S., “A new class of alkaline polymer gel electrolyte for carbon aerogel supercapacitors”,., 157, 621-623 (2006).
17 Choudhury, N.A., Shukla, A.K., Sampath, S., Pitchumani, S., “Cross-linked polymer hydrogel electrolytes for electrochemical capacitors”,..., 153, A614-A620 (2006).
18 Park, J.H., Park, O.O., Shin, K.H., Jin, C.S., Kim, J.H., “An Electrochemical capacitor based on a Ni(OH)2/activated carbon composite electrode”,.., 5, H7-H10 (2002).
19 Xu, Z.H., Wang, J.M., Shao, H.B., Zhang, J.Q., “Physical properties and electrochemical performance of solid K2FeO4samples prepared byandelectrochemical methods”,...., 15, 39-43 (2007).
20 Sun, Y.Z., Pan, J.Q., Wan, P.Y., Xu, C.C., Liu, X.G., “Electrolytic preparation, structure characterization and electrochemical performance of NiOOH”,...., 15, 262-267 (2007).
21 Lewandowski, A., Skorupska, K., Malinska, J., “Novel poly(viny alcohol)-KOH-H2O alkaline polymer electrolyte”,, 133, 265-271 (2000).
22 Yang, C.C., Lin, S.J., “Preparation of composite alkaline polymer electrolyte”,.., 57, 873-881 (2002).
23 Mohamad, A.A., Mohamed, N.S., Alias, Y., Arof, A.K., “Studies of alkaline solid polymer electrolyte and mechanically alloyed polycrystalline Mg2Ni for use in nickel metal hydride batteries”,.., 337, 208-213 (2002).
24 Mohamad, A.A., Mohamed, N.S., Yahya, M.Z.A., Othman, R., Ramesh, S., Alias, Y., Arof, A.K., “Ionic conductivity studies of poly(viny alcohol) alkaline solid polymer electrolyte and its use in nickel-zinc cells”,, 156, 171-177 (2003).
25 Zhang, G.D., Zhang, X.G., “A novel alkaline Zn/MnO2cell with alkaline solid polymer electrolyte”,, 160, 155-159 (2003).
26 Yang, C.C., “Chemical composition and XRD analyses for alkaline composite PVA polymer electrolyte”,.., 58, 33-38 (2003).
27 Yang, C.C., Lin, S.J., Hsu, S.T., “Synthesis and characterization of alkaline polyvinyl alcohol and poly(epichlorohydrin) blend polymer electrolytes and performance in electrochemical cells”,., 122, 210-218 (2003).
28 Yang, C.C., Lin, S.J., “Preparation of alkaline PVA-based polymer electrolytes for Ni-MH and Zn-air batteries”,..., 33, 777-784 (2003).
29 Palacios, I., Castillo, R., Vargas, R.A., “Thermal and transport properties of the polymer electrolyte based on poly(vinyl alcohol)-KOH-H2O”,., 48, 2195-2199 (2003).
30 Li, Z.Y., Liu, Y., Liu, H.T., He, P., Li, J.H., “Preparation and characterization of an alkaline poly(vinyl alcohol) electrolyte with high ambient conductivity”,...., 18, 625-630 (2005).
31 Liu, J.M., Wang, J.H., Yang, H.B., Zhou, Z.X., “Electrochemical stability of PVA alkaline gelpolymer electrolyte and its application in Ni/Zn secondary battery”,, 38, 37-41 (2005). (in Chinese)
32 Yang, C.C., Lin, S.J., Wu, G.M., “Study of ionic transport properties of alkaline poly(vinyl) alcohol-based polymer electrolytes”,..., 92, 251-255 (2005).
33 Yuan, A.B., Zhao, J., “Composite alkaline polymer electrolytes and its application to nickel-metal hydride batteries”,., 51, 2454-2462 (2006).
34 Saion, E., Teridi, M.A.M., “Effect of radiation on conductivity of solid PVA-KOH-PC composite polymer electrolytes”,, 12, 53-56 (2006).
35 Mohamad, A.A., Arof, A.K., “Effect of storage time on the properties of PVA-KOH alkaline solid polymer electrolyte system”,, 12, 57-61 (2006).
36 Yang, C.C., Chiu, S.J., Chien, W.C., “Development of alkaline direct methanol fuel based on crosslinked PVA polymer membranes”,., 162, 21-29 (2006).
37 Sun, Z.H., Yuan, A.B., “Preparation and properties of PVA-PAAS based alkaline polymer electrolytes”,.., 31, 804-807 (2007). (in Chinese)
38 Park, J.H., Kim, S., Park, O.O., Ko, J.M., “Improved asymmetric electrochemical capacitor using Zn-Co co-doped Ni(OH)2positive electrode material”,.., 82, 593-597 (2006).
2007-06-21,
2008-08-08.
Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50102).
** To whom correspondence should be addressed. E-mail: abyuan@shu.edu.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- An Improved Fuzzy Predictive Control Algorithm and Its Application to an Industrial CSTR Process*
- Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
- Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
- Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
- The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
- Liquid Film Characteristics on Surface of Structured Packing*
